UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K/A
AMENDMENT NO. 1
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2002 |
|
or |
|
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number 0-29889
RIGEL PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
94-3248524 (IRS Employer Identification Number) |
|
1180 Veterans Blvd. South San Francisco, California (Address of principal executive offices) |
94080 (Zip Code) |
|
(650) 624-1100 (Registrant's telephone number, including area code) |
||
Securities registered pursuant to Section 12(b) of the Act: None |
||
Securities registered pursuant to Section 12(g) of the Act: Common Stock, par value $0.001 per share (Title of Class) |
||
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by a check mark whether the registrant is an accelerated filer (as defined in Exchange Act Rule 12b-2) Yes ý No o
The approximate aggregate market value of the Common Stock held by non-affiliates of the registrant, based upon the closing price of the Common Stock as reported on the Nasdaq National Market on June 28, 2002, the last business day of the registrants most recently completed second fiscal quarter, was $100,285,535.
As of March 14, 2003, there were 45,976,828 shares of the registrant's Common Stock outstanding.
| |
|
Page |
||
|---|---|---|---|---|
| PART I | ||||
| Item 1. | Business | 1 | ||
| Item 2. | Properties | 25 | ||
| Item 3. | Legal Proceedings | 25 | ||
| Item 4. | Submission of Matters to a Vote of Security Holders | 25 | ||
PART II |
||||
| Item 5. | Market for the Registrant's Common Equity and Related Stockholder Matters | 26 | ||
| Item 6. | Selected Financial Data | 27 | ||
| Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations | 28 | ||
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 37 | ||
| Item 8. | Financial Statements and Supplementary Data | 38 | ||
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 58 | ||
PART III |
||||
| Item 10. | Directors and Executive Officers of the Registrant | 59 | ||
| Item 11. | Executive Compensation | 62 | ||
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 65 | ||
| Item 13. | Certain Relationships and Related Transactions | 68 | ||
| Item 14. | Controls and Procedures | 69 | ||
PART IV |
||||
| Item 15. | Exhibits, Financial Statement Schedules and Reports on Form 8-K | 70 | ||
| Signatures | 73 | |||
| Certifications | 75 |
i
Statements made in this document other than statements of historical fact, including statements about Rigel's scientific programs, preclinical studies, product pipeline, corporate partnerships, licenses and intellectual property, are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are subject to a number of uncertainties that could cause actual results to differ materially from the statements made, including risks associated with the success of research and product development programs, results achieved in future preclinical studies and clinical trials, the regulatory approval process, competitive technologies and products, the scope and validity of patents, proprietary technology and corporate partnerships. Reference is made to discussion about risks associated with product development programs, intellectual property and other risks that may affect our business under "Risk Factors" below. We do not undertake any obligation to update forward-looking statements.
Overview
Rigel's mission is to become a source of novel, small-molecule drugs to meet large, unmet medical needs. Our business model is to develop a portfolio of drug candidates and to take these through Phase II clinical trials, after which we intend to seek partners for completion of clinical trials, regulatory approval and marketing. We have identified three lead product development programs: mast cell inhibition to treat immunologic diseases such as asthma/allergy and autoimmune disorders, antiviral agents to treat hepatitis C, and ubiquitin ligases, a new class of cancer drug targets. We have begun clinical testing of our first product candidate, for the treatment of allergic rhinitis, and plan to begin clinical trials of two additional drug candidates for the treatment of hepatitis C and rheumatoid arthritis within the next twelve months. Our approach to drug discovery is based on advanced, proprietary functional genomics techniques that allow us to identify targets with a demonstrable role in a disease pathway and to screen efficiently for those targets that are likely to be amenable to drug modulation. We were incorporated in Delaware in June 1996, and we are based in South San Francisco, California.
Our Strategy
Our strategy is to develop a portfolio of drug candidates that can be developed into small molecule therapeutics. We believe that producing a portfolio of many drug candidates and working in conjunction with pharmaceutical companies to further develop those candidates increases our probability of commercial success. By utilizing our technology to rapidly discover and validate new targets and drug candidates in a wide range of applications, we believe that our portfolio approach allows us to minimize the risk of failure by pursuing many drug candidates at once, while concurrently being well positioned to help fill a continuing product pipeline gap of major pharmaceutical companies.
The drug development process is one that is subject to both high costs and high risk of failures. Rather than incur the costs of taking drug candidates all the way through the drug approval process and exposing ourselves to the risk of failure associated with Phase III clinical trials, we intend to identify a portfolio of new drug candidates across a broad range of diseases and develop them through Phase II clinical trials only. We believe that multiple drug candidates can be developed through Phase II clinical trials for approximately the same cost as would be required to take one drug candidate through Phase III clinical trials and marketing approval.
The key elements of our scientific and business strategy are to:
1
Proprietary Product Development
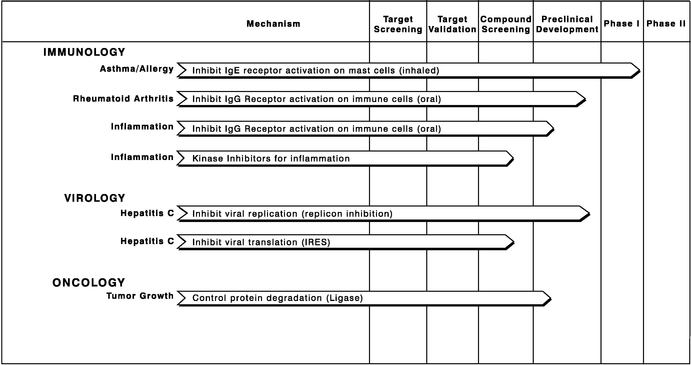
We conduct research programs for our own proprietary programs as well as for programs conducted jointly with our partners. Our proprietary programs are completely owned by us. The following table summarizes the key information for these proprietary programs that focus on specific disease mechanisms:
These Programs are:

2
Immunology
Many diseases and disorders result from defects in the immune system. Over 40 million people in the United States suffered from allergic disorders and over 20 million people suffered from asthmatic disorders in 2001. Anti-asthmatic and allergy relief medications exceeded $5 billion in worldwide sales in 2001. In 2001, another 3 million to 5 million patients in the United States were treated for other immune disorders. We currently have four programs in immunology focused on asthma/allergy, rheumatoid arthritis and inflammation.
Our mast cell kinase inhibitor program has produced a number of therapeutic opportunities. The goal of this program is to identify compounds that inhibit the secretion of inflammatory factors resulting from either IgE or IgG binding to receptors on mast cells. We believe that small molecule inhibitors of IgE or IgG signaling pathways could play an important role in the treatment of chronic immune disorders. In addition, we believe that our chemistry efforts may have identified additional kinase inhibitors that regulate other related processes within mast cells and other immune cells.
The first compound out of this program, R112, is an inhaled kinase inhibitor, and we expect that a number of additional therapeutic targets could emerge from this program.
Asthma/Allergy. We began a Phase I clinical trial of R112 in September 2002 in Britain. In this initial safety study, conducted with healthy volunteers, no significant adverse events were observed. The data from this trial was incorporated into an investigational new drug, or IND, application that was filed with the United States Food and Drug Administration, or FDA, in November 2002. Approval to proceed was received from the FDA in December 2002 and a clinical trial is now underway at National Jewish Medical Center in Denver, Colorado. The clinical trial will evaluate the effectiveness of R112 in patients with documented allergies. We expect to have the results of this study in the middle of 2003.
Rheumatoid Arthritis. Another drug candidate that we expect to emerge from our mast cell kinase inhibitor program is a compound that inhibits IgG receptor activation for therapeutic applications in the area of rheumatoid arthritis. We have administered several product candidates into animal models of rheumatoid arthritis. We expect to file an IND application with the FDA for the indication of rheumatoid arthritis by early 2004.
Inflammation. We are also researching in other autoimmune mediated inflammation disorders such as multiple sclerosis and inflammation of the bowel. We are in the process of conducting preclinical studies with our product candidates in animal models of multiple sclerosis and inflammation of the bowel.
Inflammation Using Other Targets. We have identified more than one kinase that may be inhibited in order to treat inflammation related disorders, and we are in the process of screening other compounds against various kinases in order to find additional lead compounds to potentially treat inflammation related disorders.
Virology
Experts estimate that over 170 million people worldwide are infected by the hepatitis C virus, with more than 4 million cases in the United States. Hepatitis C is a major cause of chronic hepatitis, cirrhosis and hepatocellular carcinoma. Approximately 85 percent of those who contract the disease remain chronically infected. Interferon-alpha, the current treatment standard, is ineffective in a significant portion of HCV-infected individuals, and an increasing number of patients are developing drug resistance.
Hepatits C Replicon Program. Our lead program in the hepatitis C area is a program with particular emphasis on developing a small-molecule drug candidate to block the ability of the virus to reproduce itself. This approach is substantially different from interferon-alpha, which primarily works
3
indirectly to boost the immune system. In contrast, our lead compound, R803, appears to target the virus directly by interfering with a viral protein involved in replication. R803 is currently in preclinical development, and we expect to initiate clinical trials in late 2003.
Hepatitis C IRES Program. We initiated a research program based upon technology acquired from Questcor Pharmaceuticals, Inc. in September 2000. The goal of this program is to identify compounds that interfere with the IRES translation mechanism of the hepatitis C virus. A set of high-throughput cell-based screens has been established, and initial compounds have been identified as part of this program. Under the terms of our agreement with Questcor, we are obligated to assign back to Questcor all of our rights in the technology and intellectual property to which we are entitled pursuant to the agreement if we commit a material breach of the agreement and if Questcor follows certain procedures set forth in the agreement.
Oncology
Cancer is a group of diseases characterized by the uncontrolled growth and proliferation of cells. This growth invades vital organs and often results in death. The United States market for branded cancer drugs totaled approximately $7.0 billion in 2001 and is projected to grow at an 11% annual growth rate. Cancer is the second leading cause of death in the United States, exceeded only by cardiovascular disease. In 2001, an estimated 1.3 million people were diagnosed with cancer, and more than 550,000 patients died of cancer in the United States. Although there have been improvements in cancer therapies over the last decade, there remains a significant medical need for the development of both more effective and less toxic drugs for the treatment of cancer.
Control Protein Degradation. This program is focused on characterizing and developing specific inhibitors of protein-degrading enzymes referred to as ubiquitin ligases. Many intracellular proteins that play a critical role in signaling pathways are regulated by the protein-degrading process. Many signaling proteins control cell function through active intermediates whose levels vary rapidly during different phases of a physiologic response. Disease processes can be treated by up-regulating or down-regulating these key signaling proteins as a way to enhance or dampen specific cellular responses. This antitumor program is focused on the ubiquitin ligase pathway unique to malignancies. The goal of this program is to use specific inhibitors of ubiquitin ligases that regulate mitosis, or cell division, to stop growth and induce apoptosis, or cell death, in transformed cancer cell lines. We have completed high-throughput screening, or HTS, and have identified several preclinical candidate compounds in this program. We are in the process of conducting preclinical studies.
Corporate Collaborations
We have established and will continue to pursue corporate collaborations with pharmaceutical and biotechnology companies to fund a wide array of research and development programs. We currently have collaborations with four major pharmaceutical companies, including one with Janssen Pharmaceutica N.V., a division of Johnson & Johnson, relating to oncology therapeutics and diagnostics, one with Pfizer Inc. relating to asthma and allergy therapeutics, one with Novartis Pharma AG with four different programs relating to immunology, oncology and chronic bronchitis and one with Daiichi Pharmaceuticals Co., Ltd. in the area of oncology.
As of December 31, 2002, we had received a total of $77.8 million from our collaborators. Included in this amount is $20.0 million from the private placement and public offering of equity securities and $57.8 million from the receipt of technology access fees, research funding and milestone payments, of which $6.2 million was deferred at December 31, 2002. In addition, we have a number of scientific collaborations with academic institutions and biotechnology companies under which we have in-licensed technology. We intend to pursue further collaborations as appropriate.
4
In most of our collaborations, inventions are intended to be owned by the employer of their inventors in accordance with United States patent law, subject to licenses or assignments granted in the agreements.
Johnson & Johnson
Effective December 1998, we entered into a three-year research collaboration, ended on December 4, 2001, with Johnson & Johnson, to identify, discover and validate novel drug targets that regulate cell cycle, and, specifically, to identify drug targets and the active peptides that bind to them that can restore a mutated cell's ability to stop uncontrolled cell division. In December 2001, Johnson & Johnson extended this research collaboration for an additional two years through December 2003. Under the agreement, we are providing certain assays and associated technology to Johnson & Johnson for the assessment of the alteration or normalization of the dysfunctional cell cycles of cancer cells for Johnson & Johnson's internal research purposes. Furthermore, in an amendment to the collaboration in July 2000, Johnson & Johnson expanded the collaboration whereby we performed compound screening and medicinal chemistry on some of the validated targets accepted by Johnson & Johnson. We have identified several novel drug targets in this program, four of which have been accepted by Johnson & Johnson as validated. Two of these four targets have completed HTS at Rigel and are being prepared for HTS at Johnson & Johnson.
Under the collaboration, Johnson & Johnson has the exclusive right to utilize our technology, and technology developed during the collaboration, to discover, develop, identify, make and commercialize certain products on a worldwide basis. These products are:
Johnson & Johnson also has a non-exclusive right to use our technology, and technology developed during the research collaboration, to the extent necessary to use the assays we transfer to Johnson & Johnson for internal research. Johnson & Johnson's rights are subject to its obligation to provide research funding for the collaboration, make milestone payments and technology access payments to us and pay royalties to us on the sales of products.
We will have the non-exclusive right to use any technology developed by Johnson & Johnson during the research collaboration, and any improvements to our technology developed by Johnson & Johnson during its internal research, on a royalty-free and worldwide basis.
In connection with the collaboration agreement, Johnson & Johnson Development Corporation purchased 1,500,000 shares of our Series D preferred stock at a price per share of $2.00 in connection with our Series D financing. Subsequently, Johnson & Johnson Development Corporation purchased 166,666 shares of our Series E preferred stock at a price per share of $6.00 in connection with our Series E financing. The 1,666,666 shares of preferred stock converted into 1,666,666 shares of common stock upon completion of our initial public offering in December 2000.
5
Pfizer
Effective January 1999, we entered into a research collaboration with Pfizer to identify and validate intracellular drug targets that control and inhibit the production of IgE in B Cells in the area of asthma/allergy. The research phase of the collaboration was initially scheduled to end on January 31, 2001. In January 2001, Pfizer notified us of its election to exercise its option to extend the funded research portion of the collaboration one additional year to January 31, 2002. During the research phase at Rigel, the collaboration was successful in identifying several intracellular drug targets that control the production of IgE, a key mediator in allergic reactions and asthma in B cells. Through the conclusion of the research phase of the collaboration, which was extended by one additional month to February 28, 2002, Pfizer accepted a total of seven validated targets. We believe that Pfizer has plans to move some of the validated targets forward through its drug discovery process. We have provided the following technology developed or identified during and pursuant to the research portion of the collaboration with Pfizer:
Pfizer will exclusively own drug targets for which it has initiated HTS. We will have no obligation to Pfizer with regard to any drug target Pfizer does not select for HTS.
We and Pfizer each have the non-exclusive right to use for research purposes the technology of the other that was disclosed or developed during the research collaboration, excluding our peptide libraries and proprietary cell lines. Under the collaboration, Pfizer also has the exclusive, worldwide right to develop and market diagnostic and therapeutic products for humans and animals that were identified by Pfizer in HTS and modulate the activity of a drug target identified in the research collaboration. Pfizer's rights to develop and market such products are subject to its obligation to continue to pay research milestones and subsequent royalties on the sales of these products.
At the initiation of the collaboration, Pfizer purchased 1,000,000 shares of our Series D preferred stock at a price per share of $2.00 in connection with our Series D financing, which converted into 1,000,000 shares of our common stock upon completion of our initial public offering in December 2000.
Novartis
In May 1999, we signed an agreement for the establishment of a broad collaboration with Novartis. We agreed to work with Novartis on up to five different five-year research projects to identify drug targets for products that can treat, prevent or diagnose the effects of human disease. Two of the research projects would be conducted jointly by Novartis and us, and the other three research projects were to be conducted at Novartis. The first research project, a joint research project, was focused on identifying small molecule drug targets that regulate T cells in the area of transplant rejection. The second research project, also a joint research project, related to the identification and validation of small molecule drug targets that mediate specific functions of B cells in the area of autoimmunity. During 2002, Novartis notified us that it was terminating the research phases of the initial T Cell and B Cell joint projects in November 2002 and February 2003, respectively. The third research project, a project currently being carried out at Novartis, is focused on identifying small molecule drug targets that regulate chronic bronchitis. Novartis may terminate this chronic bronchitis research at any time. In July 2001, we amended the agreement to add a three-year joint project at Rigel in the area of angiogenesis in lieu of a project at Novartis. This resulted in both funded research at Rigel and an additional upfront payment of $4.0 million, which were terms not previously included in the project at
6
Novartis. In January 2002, Novartis chose not to exercise its option to add a second project to be conducted at Novartis.
Once a drug target from any of the four ongoing research projects has been identified and validated, Novartis has the right to conduct compound screening on such drug target on an exclusive basis for two years thereafter. Novartis will have the option to extend this exclusive right for up to five additional one-year periods so long as Novartis pays us an annual fee for such right and satisfies certain diligence conditions. Upon the expiration or termination of this right, both we and Novartis shall have the non-exclusive right to use, and allow others to use, such drug target for compound screening.
Under the 1999 agreement, Novartis has the non-exclusive right to utilize our retroviral technology and pathway mapping technology for confirmational and similar uses relating to validated drug targets, including uses necessary for the further development, registration and commercialization of products for which the principal mechanism of action is based upon, derived or discovered from, or discovered with the use of, a drug target. Novartis also has the exclusive right to utilize other of our technology, and technology developed during the collaboration, to make and commercialize these products. Novartis' rights are subject to its obligation to provide research funding for the joint research projects, pay milestone payments and technology access payments to us and pay third-party royalties associated with Novartis' use of certain of our technology.
Under the agreement, we will have the non-exclusive right to use any improvements to our retroviral technology and pathway mapping technology developed during a research project on a royalty-free and worldwide basis.
Novartis purchased 2,000,000 shares of our Series D preferred stock at a per share purchase price of $2.00 in connection with our Series D financing and purchased 1,428,571 shares of our common stock in a private placement concurrent with the closing of our initial public offering at a price of $7.00 per share. The 2,000,000 shares of preferred stock converted into 2,000,000 shares of our common stock in conjunction with our initial public offering in December 2000.
Daiichi
In August 2002, we signed an agreement for the establishment of a collaboration with Daiichi to pursue research related to a specific protein degradation target. Per the agreement, the research phase of this collaboration is for three years. We will be working with Daiichi to discover and develop cancer pharmaceutical drugs. Under the terms of the collaboration agreement, Daiichi has paid us an upfront amount and a milestone payment, is obligated to pay us ongoing research support and may become obligated to pay us certain other milestones payments. In addition, we will receive royalties on any commercialized products to emerge from the collaboration.
The initial stages of the collaboration focused on the development of the assay for a specific target and the initiation of HTS to identify therapeutic molecules we and Daiichi would like to advance to later stages of drug development. Under terms of the agreement, we retain the rights to co-develop and co-promote products resulting from this collaboration in North America while Daiichi retains co-development and promotion rights in the remainder of the world.
Our Solution
The technologies that we use in connection with both our proprietary product development programs and our corporate collaborations are designed to identify protein targets for compound screening and validate the role of those targets in the disease process. Unlike genomics-based approaches, which begin by identifying genes and then search for their functions, our approach identifies proteins that are demonstrated to have an important role in a disease pathway. By understanding the disease pathway, we attempt to avoid studying genes that will not make good drug
7
targets and focus only on the sub-set of expressed proteins of genes that we believe are specifically implicated in the disease process.
We begin by developing assays that model the key events in a disease process at the cellular level. We then efficiently search hundreds of millions of cells to identify potential protein targets. In addition, we identify the proteins involved in the intracellular process and prepare a map of their interactions, thus giving us a comprehensive picture of the intracellular disease pathway. We believe that our approach has a number of advantages:
Because of the very large number of cells and proteins employed, our technology is labor intensive. The complexity of our technology requires a high degree of skill and diligence to perform successfully. In addition, successful application of our technology depends on a highly diverse collection of proteins to test in cells. We believe we have been able to and will continue to meet these challenges successfully. Although one or more other companies may utilize technologies similar to certain aspects of our technology, we are unaware of any other company that employs the same combination of technologies as we do.
Technology
Our retroviral and pathway mapping technologies enable us to identify and validate new protein targets and establish a map of the intracellular proteins that define a specific signaling pathway controlling cellular responses. We believe that, together, these technologies allow for rapid pathway mapping of complex biological processes and increase our ability to identify targets for drug discovery.
Retroviral Functional Screening. Our retroviral technology introduces up to 100 million different peptides, or proteins, into an equal number of normal or diseased cells. Each retrovirus delivers a specific gene into an individual cell, causing the cell to produce a specific protein. Then, we stimulate the cells in a manner known to produce a disease-like behavioral response or phenotype of the disease process. Once in the cell, the expressed protein interacts with potential protein targets in the cell. Then, we sort the cells at a rate of up to 60,000 cells/second to collect data on up to five different parameters, which means that a sort of 100 million cells can be completed in approximately half an hour. By analyzing the approximately 500 million resulting data points, we can rapidly identify those few cells containing an expressed protein that has interacted with a protein target in a way that causes the cell to change its behavior from diseased back to normal. Using this method, we believe that we can identify the relatively few targets that are validated in the context of a disease-specific cellular response.
8
Pathway Mapping. Our pathway mapping technology identifies specific proteins that bind with other proteins that are known to be part of a signaling pathway, either because we identified them using our retroviral technology or because the proteins have been described in the scientific literature. This pathway mapping technology is directed at:
Using our pathway mapping technology, we split a protein that gives a detectable signal (reporter protein), such as fluorescence, into two inactive parts. One part of the reporter protein is fused with a specific protein known to be involved in a signaling disease-relevant pathway (bait protein). Multiple copies of the other part of the reporter protein are fused one by one with all the proteins known to be present in the cell type being studied (library protein). When the bait protein binds to a specific library protein, the two parts of the reporter protein reunite and become active again, thereby generating a detectable signal. We employ an improved version of the two hybrid protein interaction method in yeast cells. In addition, we have developed a patented method of employing the two hybrid protein interaction technology in mammalian cells. Mammalian cells offer the opportunity to monitor protein-protein interactions in a potentially more relevant cellular environment.
We also use this pathway mapping technology to screen identified protein targets against a library of peptides in order to identify each active interaction site on the target. This information is useful in directing our chemistry efforts to identify compounds specifically designed to bind to the interaction site on the target.
Target Validation
The first step of our target validation occurs when we use our retroviral technology to identify targets. We design a screen that reflects a key event in a disease process so that when one of our proteins changes the behavior of a specific cell, this indicates a causal relationship between the protein-target interaction and the specific disease response. This approach saves time and enhances the probability that those targets that are identified and pursued are disease relevant. It also tells us that the protein interacts with a functional site on the target since the interaction results in a change in the behavior of the cell. We further validate the function of specific targets by:
Other Technologies
Our drug discovery technologies utilize the following additional technologies:
High-Throughput Compound Screening
Using our cell sorter system, we conduct screening of small molecule compounds in the same cell-based disease-specific screens that we use to identify the protein targets. This enables us to screen thousands of compounds in a matter of a few hours, while simultaneously examining multiple physiological parameters. In addition, we have established conventional high-throughput screens of small molecule compounds using biochemical methods similar to those widely used in the biotechnology
9
and pharmaceutical industries. We have a library of approximately 220,000 small molecule compounds having highly diverse molecular structures for our compound screening activities.
We select for compound screening only those protein drug targets we judge to meet several criteria:
Medicinal and Combinatorial Chemistries
Our medicinal chemistry group carries out traditional structure-activity relationship studies of potential lead compounds and makes improvements to those compounds by utilizing chemistry techniques to synthesize new analogs of a lead compound with improved properties. Our chemistry group synthesizes compounds incorporating desirable molecular features. We also utilize outside contract research organizations from time to time to supplement our internal chemistry resources.
Pharmacology and Preclinical Development
We believe that the rapid characterization and optimization of lead compounds identified in HTS will generate high-quality preclinical development candidates. Our pharmacology and preclinical development group facilitates lead optimization by characterizing lead compounds with respect to pharmacokinetics, potency, efficacy and selectivity. The generation of proof-of-principle data in animals and the establishment of standard pharmacological models with which to assess lead compounds represent integral components of lead optimization. As programs move through the lead optimization stage, our pharmacology and preclinical development group supports our chemists and biologists by performing the necessary studies, including toxicology, for IND application submissions.
Clinical Development
We have assembled a team of experts in drug development to design and implement clinical trials and to analyze the data derived from these studies. The clinical development group possesses expertise in project management and regulatory affairs.
Research and Development Expenses
Our research and development expenses were $43.4 million in 2002, $32.3 million in 2001 and $32.0 million in 2000.
Intellectual Property
We will be able to protect our technology from unauthorized use by third parties only to the extent that it is covered by valid and enforceable patents or is effectively maintained as trade secret. Accordingly, patents or other proprietary rights are an essential element of our business. We have over 100 pending patent applications and 23 issued patents in the United States which are owned or exclusively licensed in our field as well as pending corresponding foreign patent applications. Our policy is to file patent applications to protect technology, inventions and improvements to inventions that are commercially important to the development of our business. We seek United States and international patent protection for a variety of technologies, including new screening methodologies and other research tools, target molecules that are associated with disease states identified in our screens, and
10
lead compounds that can affect disease pathways. We also intend to seek patent protection or rely upon trade secret rights to protect other technologies that may be used to discover and validate targets and that may be used to identify and develop novel drugs. We seek protection, in part, through confidentiality and proprietary information agreements. We are a party to various other license agreements that give us rights to use technologies in our research and development.
In June 2002, we resolved a dispute with Inoxell A/S (formed as a spinout from Pharmexa—formally M&E Biotech) by entering into a global patent settlement concerning certain drug target identification technologies, which includes both cross-licensing and joint ownership to certain patents and allows for worldwide freedom of operation for both companies. Originally, Inoxell notified us that it had received patent protection in some European countries and Australia for a process that it asserted was similar to certain aspects of our technologies.
Competition
We face, and will continue to face, intense competition from pharmaceutical and biotechnology companies, as well as from academic and research institutions and government agencies, both in the United States and abroad. Some of these competitors are pursuing the development of pharmaceuticals that target the same diseases and conditions as our research programs. Our major competitors include fully integrated pharmaceutical companies that have extensive drug discovery efforts and are developing novel small molecule pharmaceuticals. We also face significant competition from organizations that are pursuing the same or similar technologies, including the discovery of targets that are useful in compound screening, as the technologies used by us in our drug discovery efforts. Our competitors or their collaborative partners may utilize discovery technologies and techniques or partner more rapidly or successfully than we or our collaborators are able to do.
Many of these companies and institutions, either alone or together with their collaborative partners, have substantially greater financial resources and larger research and development staffs than we do. In addition, many of these competitors, either alone or together with their collaborative partners, have significantly greater experience than we do in:
Accordingly, our competitors may succeed in obtaining patent protection, identifying or validating new targets or discovering new drug compounds before we do.
Competition may also arise from:
Developments by others may render our product candidates or technologies obsolete or noncompetitive. We face and will continue to face intense competition from other companies for collaborative arrangements with pharmaceutical and biotechnology companies, for establishing relationships with academic and research institutions and for licenses to additional technologies. These competitors, either alone or with their collaborative partners, may succeed in developing technologies or products that are more effective than ours.
11
Our ability to compete successfully will depend, in part, on our ability to:
Government Regulation
Our ongoing development activities are and will be subject to extensive regulation by numerous governmental authorities in the United States and other countries, including the FDA under the Federal Food, Drug and Cosmetic Act. The regulatory review and approval process is expensive and uncertain. Securing FDA approval requires the submission of extensive preclinical and clinical data and supporting information to the FDA for each indication to establish a product candidate's safety and efficacy. The approval process takes many years, requires the expenditure of substantial resources and may involve ongoing requirements for post-marketing studies. Clinical trials are subject to oversight by institutional review boards and the FDA and:
Even if we are able to achieve success in our clinical testing, we, or our collaborative partners, must provide the FDA and foreign regulatory authorities with clinical data that demonstrates the safety and efficacy of our products in humans before they can be approved for commercial sale. We began clinical trials in the United States in 2003, and we will not know whether these clinical trials will be successful or if such trials will be completed on schedule or at all. We also do not know whether any future clinical trials will demonstrate sufficient safety and efficacy necessary to obtain the requisite regulatory approvals or will result in marketable products. Our failure, or the failure of our strategic partners, to adequately demonstrate the safety and efficacy of our products under development will prevent receipt of FDA and similar foreign regulatory approval and, ultimately, commercialization of our products.
Any clinical trial may fail to produce results satisfactory to the FDA. Preclinical and clinical data can be interpreted in different ways, which could delay, limit or prevent regulatory approval. Negative or inconclusive results or adverse medical events during a clinical trial could cause a clinical trial to be repeated or a program to be terminated. In addition, delays or rejections may be encountered based upon additional government regulation from future legislation or administrative action or changes in FDA policy or interpretation during the period of product development, clinical trials and FDA regulatory review. Failure to comply with applicable FDA or other applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production or injunction, as well as other regulatory action against our potential
12
products, collaborative partners or us. Additionally, we have no experience in working with our partners in conducting and managing the clinical trials necessary to obtain regulatory approval.
Outside the United States, our ability to market a product is contingent upon receiving a marketing authorization from the appropriate regulatory authorities. The requirements governing the conduct of clinical trials, marketing authorization, pricing and reimbursement vary widely from country to country. At present, foreign marketing authorizations are applied for at a national level, although within the European Union, or EU, registration procedures are available to companies wishing to market a product in more than one EU member state. If the regulatory authority is satisfied that adequate evidence of safety, quality and efficacy has been presented, a marketing authorization will be granted. This foreign regulatory approval process involves all of the risks associated with FDA clearance.
Employees
As of December 31, 2002, we had 160 employees. In January 2003, we announced a restructuring of our business, and, as a result, the number of employees was reduced to 135 on January 31, 2003.
Scientific Advisors
We utilize scientists and physicians to advise us on scientific and medical matters as part of our ongoing research and drug development efforts, including experts in human genetics, mouse genetics, molecular biology, biochemistry, cell biology, chemistry, infectious diseases, immunology and structural biology. Certain of our scientific and medical advisors and consultants receive an option to purchase our common stock and an honorarium for time spent assisting us.
Executive Officers of the Registrant
See "Item 10. Directors and Executive Officers of the Registrant" in Part III hereto.
Available Information
We maintain a site on the world wide web at www.rigel.com; however, information found on our website is not incorporated by reference into this report. We make available free of charge on or through our website our annual report of Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
In 2003, we intend to adopt a code of ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. We intend to post the text of our code of ethics on our website at www.rigel.com in connection with "Investor Resources" materials. In addition, we intend to promptly disclose (1) the nature of any amendment to our code of ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions and (2) the nature of any waiver, including an implicit waiver, from a provision of our code of ethics that is granted to one of these specified officers, the name of such person who is granted the waiver and the date of the waiver on our website in the future.
13
Risk Factors
An investment in our securities is risky. Prior to making a decision about investing in our securities you should carefully consider the following risks, as well as the other information contained in this annual report on Form 10-K. If any of the following risks actually occurs, our business could be harmed. In that case, the trading price of our securities could decline, and you might lose all or part of your investment. The risks and uncertainties described below are not the only ones facing us. Additional risks and uncertainties not presently known to us, or that we currently see as immaterial, may also harm our business. If any of these additional risks or uncertainties occur, the trading price of our securities could decline, and you might lose all or part of your investment.
Our existing and committed capital resources are not sufficient to support our current operating plan beyond September 30, 2003, and we will need to obtain funding in order to continue operations beyond 2003.
We believe that our existing capital resources, together with anticipated payments under current collaborations, will be sufficient to support our current operating plan and spending through the end of September 2003. We will require additional financing to fund our operations as currently planned beyond that date. While we have been actively seeking both financing and corporate partnering opportunities, we cannot assure you that a sufficient financing or corporate partnering transaction can be completed on acceptable terms, or at all. If a sufficient financing or corporate partnering transaction cannot be completed or assured, we will not be able to continue our current operating plans and will be forced to reduce the scale of our operations. If a sufficient financing or corporate partnering transaction is not reasonably assured by the middle of May 2003, we will complete our R112 clinical trial currently under way and continue only with certain external preclinical studies in our Hepatitis C program. All other external studies would be terminated. If as of June 30, 2003 a sufficient financing or corporate partnering transaction is not reasonably assured, we will be required to significantly scale back our operations by reducing our headcount by approximately 50% and significantly reducing all discretionary spending. We anticipate that upon the execution of these actions, our existing capital resources will be sufficient to support the substantially reduced funding of our current programs as well as our operations through the end of 2003. To the extent we raise additional capital by issuing equity securities, our stockholders would at this time experience substantial dilution.
We will need additional capital in the future to sufficiently fund our operations and research.
Our operations will require significant additional funding in large part due to our research and development expenses, future preclinical and clinical-testing costs, the expansion of our facilities and the absence of any meaningful revenues for the foreseeable future. The amount of future funds needed will depend largely on the success of our collaborations and our research activities, and we do not know whether additional financing will be available when needed, or that, if available, we will obtain financing on terms favorable to our stockholders or us. We have consumed substantial amounts of capital to date, and operating expenditures are expected to increase over the next several years as we expand our infrastructure and research and development activities.
To the extent we raise additional capital by issuing equity securities, our stockholders would at this time experience substantial dilution. To the extent that we raise additional funds through collaboration and licensing arrangements, we may be required to relinquish some rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us.
Our future funding requirements will depend on many uncertain factors.
Our future funding requirements will depend upon many factors, including, but not limited to:
14
Insufficient funds may require us to delay, scale back or eliminate some or all of our research or development programs, to lose rights under existing licenses or to relinquish greater or all rights to product candidates at an earlier stage of development or on less favorable terms than we would otherwise choose or may adversely affect our ability to operate as a going concern.
Our workforce reduction announced in January 2003 and any future workforce and expense reductions may have an adverse impact on our ability to make significant progress on our internal programs.
In January 2003, we announced a workforce reduction of approximately 25 employees in order to reduce expenses. In light of our continued need for funding, we may be required to implement further workforce and expense reductions this year. Workforce and expense reductions have resulted, and further reductions could result, in reduced progress on our internal programs. In addition, employees, whether or not directly affected by a reduction, may seek future employment with our business partners or competitors. Although our employees are required to sign a confidentiality agreement at the time of hire, the confidential nature of certain proprietary information may not be maintained in the course of any such future employment. Further, we believe that our future success will depend in large part upon our ability to attract and retain highly skilled personnel. We may have difficulty attracting such personnel as a result of a perceived risk of future workforce and expense reductions. In addition, the implementation of expense reduction programs may result in the diversion of efforts of our executive management team and other key employees, which could adversely affect our business.
Our success as a company is uncertain due to our limited operating history, our history of operating losses and the uncertainty of future profitability.
Due in large part to the significant research and development expenditures required to identify and validate new drug candidates and advance our programs into clinical testing, we have not been profitable and have generated operating losses since we were incorporated in June 1996. The extent of our future losses and the timing of potential profitability are highly uncertain, and we may never achieve profitable operations. We have incurred net losses of $37.0 million, $23.8 million and $25.3 million in each of the last three fiscal years, respectively. Currently, our revenues are generated
15
solely from research payments from our collaboration agreements and licenses and are insufficient to generate profitable operations. As of December 31, 2002, we had an accumulated deficit of approximately $114.8 million. Even if we are able to secure the financing necessary to continue our operations beyond 2003, we expect to incur losses for at least the next several years and expect that these losses will increase as we expand our research and development activities, incur significant clinical and testing costs and expand our facilities.
There is a high risk that early-stage drug discovery and development might not successfully generate good drug candidates.
At the present time, the majority of our operations are in the early stages of drug identification and development. To date only one of our drug compounds has made it into the clinical testing stage. In our industry, it is statistically unlikely that the limited number of compounds that we have identified as potential drug candidates will actually lead to successful drug development efforts, and we do not expect any drugs resulting from our research to be commercially available for several years, if at all. Our one product in the clinic and our future leads for potential drug compounds will be subject to the risks and failures inherent in the development of pharmaceutical products based on new technologies. These risks include, but are not limited to, the inherent difficulty in selecting the right drug target and avoiding unwanted side effects as well as the unanticipated problems relating to product development, testing, regulatory compliance, manufacturing, marketing, competition and costs and expenses that may exceed current estimates.
We might not be able to commercialize our drug candidates successfully if problems arise in the clinical testing and approval process.
Commercialization of our product candidates depends upon successful completion of preclinical studies and clinical trials. Preclinical testing and clinical development are long, expensive and uncertain processes. We do not know whether we, or any of our collaborative partners, will be permitted to undertake clinical trials of potential products beyond the one trial already concluded and the trial currently in process. It may take us or our collaborative partners several years to complete any such testing, and failure can occur at any stage of testing. Interim results of trials do not necessarily predict final results, and acceptable results in early trials may not be repeated in later trials. A number of companies in the pharmaceutical industry, including biotechnology companies, have suffered significant setbacks in advanced clinical trials, even after achieving promising results in earlier trials. Moreover, when our projects reach clinical trials, we or our collaborative partners may decide to discontinue development of any or all of these projects at any time for commercial, scientific or other reasons.
Delays in clinical testing could result in increased costs to us.
Significant delays in clinical testing could materially impact our product development costs. We do not know whether planned clinical trials will begin on time, will need to be revamped or will be completed on schedule, or at all. Clinical trials can be delayed for a variety of reasons, including delays in obtaining regulatory approval to commence a study, delays in reaching agreement on acceptable clinical study agreement terms with prospective clinical sites, delays in obtaining institutional review board approval to conduct a study at a prospective clinical site or delays in recruiting subjects to participate in a study.
In addition, we typically rely on third-party clinical investigators to conduct our clinical trials and other third-party organizations to oversee the operations of such trials and to perform data collection and analysis. As a result, we may face additional delaying factors outside our control if these parties do not perform their obligations in a timely fashion. While we have not yet experienced delays that have materially impacted our clinical trials or product development costs, delays of this sort could occur for the reasons identified above or other reasons. If we have delays in testing or approvals, our product
16
development costs will increase. For example, we may need to make additional payments to third-party investigators and organizations to retain their services or we may need to pay recruitment incentives. If the delays are significant, our financial results and the commercial prospects for our product candidates will be harmed, and our ability to become profitable will be delayed.
Because most of our expected future revenues are contingent upon collaborative and license agreements, we might not meet our strategic objectives.
Our ability to generate revenues in the near term depends on our ability to enter into additional collaborative agreements with third parties and to maintain the agreements we currently have in place. Our ability to enter into new collaborations and the revenue, if any, that may be recognized under these collaborations is highly uncertain. If we are unable to enter into new collaborations, our business prospects could be harmed, which could have an immediate adverse effect on the trading price of our stock.
To date, most of our revenues have been related to the research phase of each of our collaborative agreements. Such revenues are for specified periods, and the impact of such revenues on our results of operations is partially offset by corresponding research costs. Following the completion of the research phase of each collaborative agreement, additional revenue may come only from milestone payments and royalties, which may not be paid, if at all, until some time well into the future. The risk is heightened due to the fact that unsuccessful research efforts may preclude us from receiving any milestone payments under these agreements. Our receipt of revenue from collaborative arrangements is also significantly affected by the timing of efforts expended by us and our collaborators and the timing of lead compound identification. In late 2001, we recorded the first revenue from achievement of milestones in both the Pfizer and Johnson & Johnson collaborations. During 2002, we recorded our first milestone for both Novartis and Daiichi. Under many agreements, however, milestone payments may not be earned until the collaborator has advanced products into clinical testing, which may never occur or may not occur until some time well into the future. If we are not able to recognize revenue under our collaborations when and in accordance with our expectations or the expectations of industry analysts, this failure could harm our business and have an immediate adverse effect on the trading price of our stock.
Our business requires us to generate meaningful revenue from royalties and licensing agreements. To date, we have not received any revenue from royalties for the commercial sale of drugs, and we do not know when we will receive any such revenue, if at all. Likewise, we have not licensed any lead compounds or drug development candidates to third parties, and we do not know whether any such license will be entered into on acceptable terms in the future, if at all.
If our current corporate collaborations or license agreements are unsuccessful our research and development efforts could be delayed.
Our strategy depends upon the formation and sustainability of multiple collaborative arrangements and license agreements with third parties in the future. We rely on these arrangements for not only financial resources, but also for expertise that we expect to need in the future relating to clinical trials, manufacturing, sales and marketing, and for licenses to technology rights. To date, we have entered into several such arrangements with corporate collaborators; however, we do not know if such third parties will dedicate sufficient resources or if any development or commercialization efforts by third parties will be successful. Should a collaborative partner fail to develop or commercialize a compound or product to which it has rights from us, such failure might delay ongoing research and development efforts at Rigel because we might not receive any future milestone payments and we will not receive any royalties associated with such compound or product. In addition, the continuation of some of our partnered drug discovery and development programs may be dependent on the periodic renewal of our corporate collaborations. For example, the funded research phase of our collaboration with Pfizer has been
17
completed and the development portion of our collaboration is ongoing at Pfizer. In addition, in May 2002, Novartis elected to conclude the research phases of our two initial joint projects in the autoimmunity and transplant rejection areas, after 42 months, effective November 2002 and February 2003, respectively. Pursuant to the collaboration agreement, Novartis had the option to end the research phase on these programs after 24 months or 42 months. More generally, our current corporate collaboration agreements may terminate upon a breach or a change of control. We may not be able to renew these collaborations on acceptable terms, if at all, or negotiate additional corporate collaborations on acceptable terms, if at all.
Conflicts also might arise with collaborative partners concerning proprietary rights to particular compounds. While our existing collaborative agreements typically provide that we retain milestone payments and royalty rights with respect to drugs developed from certain derivative compounds, any such payments or royalty rights may be at reduced rates, and disputes may arise over the application of derivative payment provisions to such drugs, and we may not be successful in such disputes.
We are also a party to various license agreements that give us rights to use specified technologies in our research and development processes. The agreements pursuant to which we have in-licensed technology permit our licensors to terminate the agreements under certain circumstances If we are not able to continue to license these and future technologies on commercially reasonable terms, our product development and research may be delayed.
If conflicts arise between our collaborators or advisors and us, any of them may act in their self-interest, which may be adverse to your interests.
If conflicts arise between us and our corporate collaborators or scientific advisors, the other party may act in its self-interest and not in the interest of our stockholders. Some of our corporate collaborators are conducting multiple product development efforts within each disease area that is the subject of the collaboration with us. In some of our collaborations, we have agreed not to conduct, independently or with any third party, any research that is competitive with the research conducted under our collaborations. Our collaborators, however, may develop, either alone or with others, products in related fields that are competitive with the products or potential products that are the subject of these collaborations. Competing products, either developed by our collaborators or to which our collaborators have rights, may result in their withdrawal of support for our product candidates.
If any of our corporate collaborators were to breach or terminate its agreement with us or otherwise fail to conduct the collaborative activities successfully and in a timely manner, the preclinical or clinical development or commercialization of the affected product candidates or research programs could be delayed or terminated. We generally do not control the amount and timing of resources that our corporate collaborators devote to our programs or potential products. We do not know whether current or future collaborative partners, if any, might pursue alternative technologies or develop alternative products either on their own or in collaboration with others, including our competitors, as a means for developing treatments for the diseases targeted by collaborative arrangements with us.
If we fail to enter into new collaborative arrangements in the future, our business and operations would be negatively impacted.
Although we have established several collaborative arrangements and various license agreements, we do not know if we will be able to establish additional arrangements in the future. For example, there have been, and may continue to be, a significant number of recent business combinations among large pharmaceutical companies that have resulted, and may continue to result, in a reduced number of potential future corporate collaborators, which may limit our ability to find partners who will work with us in developing and commercializing our drug targets. We entered into only one collaboration, with Daiichi, in 2002. If business combinations involving our existing corporate collaborators were to occur,
18
the effect could be to diminish, terminate or cause delays in one or more of our corporate collaborations.
Our success is dependent on intellectual property rights held by us and third parties, and our interest in such rights is complex and uncertain.
Our success will depend to a large part on our own, our licensees' and our licensors' ability to obtain and defend patents for each party's respective technologies and the compounds and other products, if any, resulting from the application of such technologies. We have over 100 pending patent applications and 23 issued patents in the United States that are owned or exclusively licensed in our field as well as pending corresponding foreign patent applications. In the future, our patent position might be highly uncertain and involve complex legal and factual questions. Additional uncertainty may result from because no consistent policy regarding the breadth of legal claims allowed in biotechnology patents has emerged to date. Accordingly, we cannot predict the breadth of claims allowed in our or other companies' patents.
Because the degree of future protection for our proprietary rights is uncertain, we cannot ensure that:
We rely on trade secrets to protect technology where we believe patent protection is not appropriate or obtainable. However, trade secrets are difficult to protect. While we require employees, collaborators and consultants to enter into confidentiality agreements, we may not be able to adequately protect our trade secrets or other proprietary information in the event of any unauthorized use or disclosure or the lawful development by others of such information.
We are a party to certain in-license agreements that are important to our business, and we generally do not control the prosecution of in-licensed technology. Accordingly, we are unable to exercise the same degree of control over this intellectual property as we exercise over our internally-developed technology. Moreover, some of our academic institution licensors, research collaborators and scientific advisors have rights to publish data and information in which we have rights. If we cannot maintain the confidentiality of our technology and other confidential information in connection with our collaborations, then our ability to receive patent protection or protect our proprietary information will be impaired. In addition, some of the technology we have licensed relies on patented inventions developed using U.S. government resources. The U.S. government retains certain rights, as defined by law, in such patents, and may choose to exercise such rights.
19
If a dispute arises regarding the infringement or misappropriation of the proprietary rights of others, such dispute could be costly and result in delays in our research and development activities.
Our success will also depend, in part, on our ability to operate without infringing or misappropriating the proprietary rights of others. There are many issued patents and patent applications filed by third parties relating to products or processes that are similar or identical to ours or our licensors, and others may be filed in the future. There can be no assurance that our activities, or those of our licensors, will not infringe patents owned by others. For example, in June 2002, we resolved a dispute with Inoxell A/S (formed as a spinout from Pharmexa—formally M&E Biotech) by entering into a global patent settlement concerning certain drug target identification technologies, which includes both cross-licensing and joint ownership to certain patents and allows for worldwide freedom of operation for both companies. We believe that there may be significant litigation in the industry regarding patent and other intellectual property rights, and we do not know if we or our collaborators would be successful in any such litigation. Any legal action against our collaborators or us claiming damages or seeking to enjoin commercial activities relating to the affected products, our methods or processes could:
If we are unable to obtain regulatory approval to market products in the United States and foreign jurisdictions, we might not be permitted to commercialize products from our research and development.
Due, in part, to the early stage of our drug candidate research and development process, we cannot predict whether regulatory clearance will be obtained for any product that we, or our collaborative partners, hope to develop. Satisfaction of regulatory requirements typically takes many years, is dependent upon the type, complexity and novelty of the product and requires the expenditure of substantial resources. Of particular significance to us are the requirements covering research and development and testing.
Before commencing clinical trials in humans in the United States, we, or our collaborative partners, will need to submit and receive approval from the FDA of an IND. Clinical trials are subject to oversight by institutional review boards and the FDA and:
20
While we have stated that we intend to file additional INDs, this is only a statement of intent, and we may not be able to do so because we may not be able to identify potential product candidates. In addition, the FDA may not approve any IND in a timely manner, or at all.
Before receiving FDA clearance to market a product, we must demonstrate that the product is safe and effective on the patient population that will be treated. Data obtained from preclinical and clinical activities are susceptible to varying interpretations that could delay, limit or prevent regulatory clearances. In addition, delays or rejections may be encountered based upon additional government regulation from future legislation or administrative action or changes in FDA policy during the period of product development, clinical trials and FDA regulatory review. Failure to comply with applicable FDA or other applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production or injunction, as well as other regulatory action against our potential products or us. Additionally, we have limited experience in conducting and managing the clinical trials necessary to obtain regulatory approval.
If regulatory clearance of a product is granted, this clearance will be limited to those disease states and conditions for which the product is demonstrated through clinical trials to be safe and efficacious. We cannot ensure that any compound developed by us, alone or with others, will prove to be safe and efficacious in clinical trials and will meet all of the applicable regulatory requirements needed to receive marketing clearance.
Outside the United States, our ability, or that of our collaborative partners, to market a product is contingent upon receiving a marketing authorization from the appropriate regulatory authorities. This foreign regulatory approval process typically includes all of the risks associated with FDA clearance described above and may also include additional risks.
If our competitors develop technologies that are more effective than ours, our commercial opportunity will be reduced or eliminated.
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. Many of the drugs that we are attempting to discover will be competing with existing therapies. In addition, a number of companies are pursuing the development of pharmaceuticals that target the same diseases and conditions that we are targeting. We face competition from pharmaceutical and biotechnology companies both in the United States and abroad.
Our competitors may utilize discovery technologies and techniques or partner with collaborators in order to develop products more rapidly or successfully than we, or our collaborators, are able to do. Many of our competitors, particularly large pharmaceutical companies, have substantially greater financial, technical and human resources than we do. In addition, academic institutions, government agencies and other public and private organizations conducting research may seek patent protection with respect to potentially competitive products or technologies and may establish exclusive collaborative or licensing relationships with our competitors.
We believe that our ability to compete is dependent, in part, upon our ability to create, maintain and license scientifically-advanced technology and upon our and our strategic partners' ability to develop and commercialize pharmaceutical products based on this technology, as well as our ability to attract and retain qualified personnel, obtain patent protection or otherwise develop proprietary technology or processes and secure sufficient capital resources for the expected substantial time period between technological conception and commercial sales of products based upon our technology. The failure by us or any of our collaborators in any of those areas may prevent the successful commercialization of our potential drug targets.
Our competitors might develop technologies and drugs that are more effective or less costly than any that are being developed by us or that would render our technology and potential drugs obsolete
21
and noncompetitive. In addition, our competitors may succeed in obtaining the approval of the FDA or other regulatory agencies for drug candidates more rapidly. Companies that complete clinical trials, obtain required regulatory agency approvals and commence commercial sale of their drugs before their competitors may achieve a significant competitive advantage, including certain patent and FDA marketing exclusivity rights that would delay or prevent our ability to market certain products. Any drugs resulting from our research and development efforts, or from our joint efforts with our existing or future collaborative partners, might not be able to compete successfully with competitors' existing or future products or products under development or obtain regulatory approval in the United States or elsewhere.
Our ability to generate revenues will be diminished if our collaborative partners fail to obtain acceptable prices or an adequate level of reimbursement for products from third-party payors.
The drugs we hope to develop may be rejected by the marketplace due to many factors, including cost. Our ability to commercially exploit a drug may be limited due to the continuing efforts of government and third-party payors to contain or reduce the costs of health care through various means. For example, in some foreign markets, pricing and profitability of prescription pharmaceuticals are subject to government control. In the United States, we expect that there will continue to be a number of federal and state proposals to implement similar government control. In addition, increasing emphasis on managed care in the United States will likely continue to put pressure on the pricing of pharmaceutical products. Cost control initiatives could decrease the price that any of our collaborators would receive for any products in the future. Further, cost control initiatives could adversely affect our collaborators' ability to commercialize our products and our ability to realize royalties from this commercialization.
Our ability to commercialize pharmaceutical products with collaborators may depend, in part, on the extent to which reimbursement for the products will be available from:
Significant uncertainty exists as to the reimbursement status of newly-approved healthcare products. Third-party payors, including Medicare, are challenging the prices charged for medical products and services. Government and other third-party payors increasingly are attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for new drugs and by refusing, in some cases, to provide coverage for uses of approved products for disease indications for which the FDA has not granted labeling approval. Third-party insurance coverage may not be available to patients for any products we discover and develop, alone or with collaborators. If government and other third-party payors do not provide adequate coverage and reimbursement levels for our products, the market acceptance of these products may be reduced.
If product liability lawsuits are successfully brought against us, we may incur substantial liabilities and may be required to limit commercialization of our products.
The testing and marketing of medical products entail an inherent risk of product liability. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our products. We currently do not have product liability insurance, and our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of pharmaceutical products we develop, alone or with corporate collaborators. We, or our corporate collaborators, might not be able to obtain insurance at a reasonable cost, if at all. While under various
22
circumstances we are entitled to be indemnified against losses by our corporate collaborators, indemnification may not be available or adequate should any claim arise.
Our research and development efforts will be seriously jeopardized, if we are unable to attract and retain key employees and relationships.
As a small company with only 135 employees as of January 31, 2003, our success depends on the continued contributions of our principal management and scientific personnel and on our ability to develop and maintain important relationships with leading academic institutions, scientists and companies in the face of intense competition for such personnel. In particular, our research programs depend on our ability to attract and retain highly skilled chemists, other scientists, and regulatory and clinical personnel. If we lose the services of any of our personnel, our research and development efforts could be seriously and adversely affected. Our employees can terminate their employment with us at any time.
We depend on various scientific consultants and advisors for the success and continuation of our research efforts.
We work extensively with various scientific consultants and advisors. The potential success of our drug discovery programs depends, in part, on continued collaborations with certain of these consultants and advisors. We, and various members of our management and research staff, rely on certain of these consultants and advisors for expertise in our research, regulatory and clinical efforts. Our scientific advisors are not employees of ours and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. We do not know if we will be able to maintain such consulting agreements or that such scientific advisors will not enter into consulting arrangements, exclusive or otherwise, with competing pharmaceutical or biotechnology companies, any of which would have a detrimental impact on our research objectives and could have a material adverse effect on our business, financial condition and results of operations.
If we use biological and hazardous materials in a manner that causes injury or violates laws, we may be liable for damages.
Our research and development activities involve the controlled use of potentially harmful biological materials as well as hazardous materials, chemicals and various radioactive compounds. We cannot completely eliminate the risk of accidental contamination or injury from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for damages that result, and such liability could exceed our resources. We are subject to federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. The cost of compliance with, or any potential violation of, these laws and regulations could be significant.
Our facilities are located near known earthquake fault zones, and the occurrence of an earthquake or other catastrophic disaster could cause damage to our facilities and equipment, which could require us to cease or curtail operations.
Our facilities are located in the San Francisco Bay Area near known earthquake fault zones and are vulnerable to significant damage from earthquakes. We are also vulnerable to damage from other types of disasters, including fires, floods, power loss, communications failures and similar events. If any disaster were to occur, our ability to operate our business at our facilities would be seriously, or potentially completely, impaired, and our research could be lost or destroyed. In addition, the unique nature of our research activities and of much of our equipment could make it difficult for us to recover from a disaster. The insurance we maintain may not be adequate to cover or losses resulting from disasters or other business interruptions.
23
If our officers, directors and largest stockholders choose to act together, they may be able to significantly affect our management and operations, acting in their best interests and not necessarily those of other stockholders.
Our directors, executive officers and principal stockholders and their affiliates beneficially own approximately 47% of our common stock, based on their beneficial ownership as of February 15, 2003. Accordingly, they collectively will have the ability to significantly affect the election of all of our directors and the outcome of most corporate actions requiring stockholder approval. They may exercise this ability in a manner that advances their best interests and not necessarily those of other stockholders.
Our common stock may be delisted from Nasdaq.
Since January 22, 2003, the closing price of our common stock has been below $1.00 for greater than 30 consecutive business days. On March 7, 2003, we received written notice from Nasdaq that we have failed to maintain the minimum closing bid price of $1.00 for 30 consecutive business days as required by the Nasdaq National Market. If we are unable to demonstrate compliance with this or any other Nasdaq requirement, Nasdaq may take further action with respect to a potential delisting of our common stock. We may appeal any such decision by Nasdaq to the Nasdaq Listing Qualifications Panel. If our common stock were delisted from the Nasdaq National Market this could result, among other things, in a number of negative implications, including reduced liquidity in our common stock as a result of the loss of market efficiencies associated with the Nasdaq National Market, as well as the potential loss of confidence by suppliers, collaborators and employees, the loss of analyst coverage and institutional investor interest, fewer business development opportunities and greater difficulty in obtaining financing.
Our stock price may be volatile, and your investment in our stock could decline in value.
The market prices for our securities and those of other biotechnology companies have been highly volatile and may continue to be highly volatile in the future. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our common stock:
24
Anti-takeover provisions in our charter documents and under Delaware law may make an acquisition of us, which may be beneficial to our stockholders, more difficult.
Provisions of our amended and restated certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us, even if doing so would benefit our stockholders. These provisions:
In addition, Section 203 of the Delaware General Corporation Law, which imposes certain restrictions relating to transactions with major stockholders, may discourage, delay or prevent a third party from acquiring us.
Our current facilities consist of approximately 147,000 square feet of research and office space located at 1180 Veterans Boulevard, South San Francisco, California. We believe our facilities are in good operating condition and that the real property leased is adequate for all present and near term uses.
None.
Item 4. Submission of Matters to a Vote of Security Holders
None.
25
Item 5. Market for the Registrant's Common Equity and Related Stockholder Matters
Our common stock has traded on the Nasdaq National Market under the symbol "RIGL" since November 29, 2000. The following table sets forth, for the periods indicated, the high and low sales prices for the common stock as reported by the Nasdaq National Market:
| |
High |
Low |
|||||
|---|---|---|---|---|---|---|---|
| Year Ended December 31, 2001 | |||||||
| First Quarter | $ | 12.75 | $ | 3.38 | |||
| Second Quarter | $ | 8.50 | $ | 3.25 | |||
| Third Quarter | $ | 8.75 | $ | 4.00 | |||
| Fourth Quarter | $ | 6.42 | $ | 4.00 | |||
| Year Ended December 31, 2002 | |||||||
| First Quarter | $ | 5.10 | $ | 3.40 | |||
| Second Quarter | $ | 4.83 | $ | 2.20 | |||
| Third Quarter | $ | 2.97 | $ | 1.41 | |||
| Fourth Quarter | $ | 1.90 | $ | 1.05 | |||
On March 14, 2003, the last reported sale price for our common stock on the Nasdaq National Market was $0.65 per share.
Holders
As of March 14, 2003, there were approximately 224 stockholders of record of our common stock.
Dividends
We have not paid dividends on our common stock and currently do not plan to pay any cash dividends in the foreseeable future.
Securities Authorized for Issuance Under Equity Compensation Plans
Please See Part III, Item 12, page 66, for information with respect to an equity compensation plan adopted without the approval of our stockholders.
Sales of Unregistered Securities
In conjunction with the amendment of our master lease agreement for our 1180 Veterans Blvd. facility entered into in October 2002, we issued a warrant to purchase 500,000 shares of our common stock at an exercise price of $1.97 per share to Kwacker Limited. This warrant will expire on October 18, 2007. The warrant was issued in a private transaction pursuant to an exemption from registration in reliance upon Section 4(2) of the Securities Act of 1934, as amended. In conjunction with this amendment, we also amended the terms of an outstanding warrant that had been issued to Kwacker Limited in May 2001 to purchase 150,000 shares of our common stock at an exercise price of $8.91 per share. This warrant was amended and restated into the form of the new warrant issued in October 2002.
In conjunction with the equipment lease line executed in December 2002, we issued a warrant to purchase 186,916 shares of our common stock at an exercise price of $1.07 per share to Lighthouse Capital Partners IV, L.P. This warrant will expire on December 23, 2007. The warrant was issued in a private transaction pursuant to an exemption from registration in reliance upon Section 4(2) of the Securities Act of 1934, as amended.
26
Item 6. Selected Financial Data
The following selected financial data should be read in conjunction with "Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Item 8. Financial Statements and Supplementary Data" included elsewhere in this annual report on Form 10-K.
| |
Fiscal Years Ended December 31, |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2002 |
2001 |
2000 |
1999 |
1998 |
||||||||||||
| |
(in thousands, except per share amounts) |
||||||||||||||||
| Statements of Operations Data: | |||||||||||||||||
| Contract revenues | $ | 15,788 | $ | 15,303 | $ | 13,218 | $ | 8,984 | $ | 28 | |||||||
| Costs and expenses: | |||||||||||||||||
| Research and development (see Note A) | 43,350 | 32,313 | 32,034 | 17,112 | 8,305 | ||||||||||||
| General and administrative (see Note A) | 9,454 | 7,950 | 6,689 | 3,952 | 2,217 | ||||||||||||
| 52,804 | 40,263 | 38,723 | 21,064 | 10,522 | |||||||||||||
| Loss from operations | (37,016 | ) | (24,960 | ) | (25,505 | ) | (12,080 | ) | (10,494 | ) | |||||||
| Interest income | 856 | 1,957 | 1,078 | 311 | 246 | ||||||||||||
| Interest expense | (870 | ) | (802 | ) | (933 | ) | (597 | ) | (356 | ) | |||||||
| Net loss | (37,030 | ) | (23,805 | ) | (25,360 | ) | (12,366 | ) | (10,604 | ) | |||||||
| Deemed dividend to Series E preferred stockholders | — | — | (10,133 | ) | — | — | |||||||||||
| Net loss allocable to common stockholders | $ | (37,030 | ) | $ | (23,805 | ) | $ | (35,493 | ) | $ | (12,366 | ) | $ | (10,604 | ) | ||
| Net loss per share, basic and diluted | $ | (0.82 | ) | $ | (0.64 | ) | $ | (4.89 | ) | $ | (4.39 | ) | $ | (4.01 | ) | ||
| Weighted average shares used in computing net loss per share, basic and diluted | 44,954 | 37,287 | 7,263 | 2,818 | 2,643 | ||||||||||||
| Pro forma net loss per share, basic and diluted | $ | (0.86 | ) | $ | (0.52 | ) | |||||||||||
| Shares used in computing pro forma net loss per share, basic and diluted | 29,543 | 23,996 | |||||||||||||||
Note A: |
|||||||||||||||||
| Includes charges for stock-based compensation as follows: | |||||||||||||||||
| Research and development | $ | 568 | $ | 1,596 | $ | 9,184 | $ | 2,321 | $ | 6 | |||||||
| General and administrative | 191 | 527 | 976 | 254 | — | ||||||||||||
| Total stock-based compensation | $ | 759 | $ | 2,123 | $ | 10,160 | $ | 2,575 | $ | 6 | |||||||
| |
As of December 31, |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2002 |
2001 |
2000 |
1999 |
1998 |
|||||||||||
| |
(in thousands) |
|||||||||||||||
| Balance Sheet Data: | ||||||||||||||||
| Cash, cash equivalents and available-for-sale securities | $ | 27,291 | $ | 33,415 | $ | 52,994 | $ | 5,836 | $ | 9,493 | ||||||
| Working capital (deficiency) | 22,493 | 26,371 | 46,627 | (990 | ) | 4,547 | ||||||||||
| Total assets | 44,342 | 46,448 | 64,262 | 17,169 | 12,956 | |||||||||||
| Capital lease obligations, less current portion | 2,313 | 4,243 | 5,761 | 5,478 | 1,652 | |||||||||||
| Deferred stock compensation | (772 | ) | (2,452 | ) | (5,792 | ) | (5,814 | ) | — | |||||||
| Accumulated deficit | (114,814 | ) | (77,784 | ) | (53,979 | ) | (28,619 | ) | (16,253 | ) | ||||||
| Total stockholders' equity | 25,441 | 28,941 | 49,010 | 756 | 5,445 | |||||||||||
See Notes to the Financial Statements for description of the number of shares used in the computation of basic and diluted and pro forma basic and diluted net loss per common share.
27
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis contains forward-looking statements that are based upon current expectations. Forward-looking statements involve risks and uncertainties. Our actual results and the timing of events may differ significantly from the results discussed in the forward-looking statements. Factors that might cause such a difference include, but are not limited to, those discussed in "Business—Risk Factors" as well as those discussed elsewhere in this annual report on Form 10-K. Historical operating results are not necessarily indicative of results that may occur in future periods. You should read the following discussion and analysis in conjunction with "Item 6. Selected Financial Data," and "Item 8. Financial Statements and Supplementary Data" included elsewhere in this annual report on Form 10-K.
Overview
Our mission is to become a source of novel, small-molecule therapeutic drugs to meet large, unmet medical needs. Our business model is to develop a portfolio of drug candidates and to take these through phase II clinical trials, after which we intend to seek partners for completion of clinical trials, regulatory approval and marketing. We have incurred net losses since inception and expect to incur substantial and increasing losses for the next several years as we continue to move drug candidates into and through preclinical and clinical stages of drug development and expand our research and development activities. To date, we have funded our operations primarily through the sale of equity securities, non-equity payments from collaborative partners and capital asset lease financings. We received our first funding from our collaborative partners in December 1998. As of December 31, 2002, including both research funding and equity investments, we had received an aggregate of $77.8 million from our collaborative partners, including $15.7 million in the year ended December 31, 2002. As of December 31, 2002, our accumulated deficit was approximately $114.8 million.
We expect our sources of revenue for the next several years to consist primarily of payments under our current and future corporate collaborations. Under these arrangements, sources of revenue may include up-front payments, funded research, milestone payments and royalties. The process of carrying out our research programs for our collaborative partners and the development of our own non-partnered products to the later stages of development will require significant additional research and development expenditures, including preclinical testing and clinical trials. These activities, together with our general and administrative expenses, are expected to result in substantial operating losses for the foreseeable future. We will not receive product revenue unless we or our collaborative partners complete clinical trials, obtain regulatory approval and successfully commercialize one or more of our products.
To date, we have entered into collaborations with four major pharmaceutical companies: Johnson & Johnson, Pfizer, Novartis and Daiichi. Johnson & Johnson, Pfizer and Novartis have contributed nearly all of our revenues over the last three years. The Daiichi collaboration was entered into in the last half of 2002.
In July 2001, we expanded our collaboration with Novartis with the initiation of our angiogenesis program, the fourth and final program in our Novartis collaboration. Pursuant to the expanded Novartis collaboration, we received a $4.0 million up-front payment from Novartis, which is being recognized as revenue ratably through July 2004. In addition, the expanded collaboration provides that the angiogenesis research program will be carried out at Rigel, provides for research reimbursement through the middle of 2004 and includes potential future milestones and royalty payments to us. In conjunction with the original collaboration, Novartis paid $4.0 million for 2,000,000 shares of our series D preferred stock that converted to 2,000,000 shares of common stock upon the completion of our initial public offering. The original collaboration also allowed for an additional equity investment by Novartis of up to $10.0 million that was callable by us until our initial public offering. We exercised
28
this right and sold to Novartis 1,428,571 shares of common stock at $7.00 per share concurrent with the closing of the our initial public offering.
In May 2002, Novartis elected to conclude the research phases of our two initial joint projects in the autoimmunity and transplant rejection areas, after 42 months each, effective in November 2002 and February 2003, respectively.
In December 2001, Johnson & Johnson elected to extend the research phase of our collaboration for an additional two years, and we estimate that this extension will result in additional research reimbursement through the end of 2003 of approximately $5.0 million, of which $2.5 million has been received as of December 31, 2002.
In February 2002, the research phase of our collaboration with Pfizer concluded with Pfizer accepting a total of seven validated targets. Under our collaboration with Pfizer, we expect that these validated targets will continue through the drug discovery and development process at Pfizer.
In August 2002, we signed an agreement for the establishment of a collaboration with Daiichi to pursue research related to a specific protein degradation target. Per the agreement, the research phase of this collaboration is for three years. We will be working with Daiichi to discover and develop cancer pharmaceutical drugs. Under the terms of the collaboration agreement, Daiichi has paid us an upfront amount and a milestone payment, is obligated to pay us ongoing research support and may become obligated to pay us certain other milestones payments. In addition, we will receive royalties on any commercialized products to emerge from the collaboration.
The initial stages of the collaboration focused on the development of the assay for a specific target and the initiation of HTS to identify therapeutic molecules we and Daiichi would like to advance to later stages of drug development. Under terms of the agreement, we retain the rights to co-develop and co-promote products resulting from this collaboration in North America while Daiichi retains co-development and promotion rights in the remainder of the world.
A summary of these partnerships is as follows:
| Partner |
Research Program |
Commencement Date |
Research Phase Termination Date |
|||
|---|---|---|---|---|---|---|
| Johnson & Johnson | Tumor Growth—Cell Cycle Inhibition | December 4, 1998 | December 2003 | |||
| Pfizer | Asthma/Allergy—IgE Production in B Cells | January 31, 1999 | February 2002 | |||
| Novartis | Transplant Rejection—T Cell Activation | May 26, 1999 | November 2002 | |||
| Novartis | Autoimmunity Disease—B Cell Activation | August 1, 1999 | February 2003 | |||
| Novartis | Chronic Bronchitis (conducted at Novartis) | January 1, 2000 | Ongoing at Novartis | |||
| Novartis | Tumor Growth—Inhibition of Tumor Angiogenesis | July 6, 2001 | July 2004 | |||
| Daiichi | Tumor Growth—Protein Degradation Oncology Target | August 1, 2002 | August 2005 |
Under the terms of these collaborations, Johnson & Johnson, Novartis and Daiichi have agreed to provide up to approximately $10.3 million in future research funding over the next three years, none of which is cancelable at the option of these partners. In addition, we may receive additional payments upon the achievement of specific research and development milestones and royalties upon commercialization of any products.
29
In order to maintain and increase proceeds from collaborations, we are exploring new opportunities with existing and new potential collaborators. Our earliest partnerships focused on the early stages of drug discovery, specifically on target discovery and validation, while our collaboration with Johnson & Johnson has been expanded to also include both chemistry and compound HTS, and our recent collaboration with Daiichi focuses on drug discovery and development. We currently anticipate that in order to support our current research programs we will need to self-fund, at an increased rate of spending, our own research programs to later stages of development prior to partnering with collaborative partners. Therefore, it is expected that future collaborative partnerships may have an expanded focus and could include HTS, combinatorial and medicinal chemistry, preclinical evaluations and/or clinical development. For some programs, we may also seek to enter into collaborations for the development of compounds that we have discovered. The timing, the amount of funds received and the scope of any new collaborations are uncertain, and any compound collaboration will depend on the successful progress of clinical trials. New, expanded or larger collaborations will also be necessary to offset any decrease in proceeds as collaborations come to the end of their terms. Our remaining Novartis program focused on angiogenesis is a multiple-year agreement with the research phase terminating in 2004, the Johnson & Johnson collaboration concludes its research phase at the end of 2003 and the Daiichi collaboration concludes its research phase in August 2005. As each collaboration reaches the conclusion of its research phase, the parties may evaluate the status of the collaboration and, if appropriate, seek to extend the research phase of the collaboration agreement or negotiate alternative terms.
In June 2002, we resolved a dispute with Inoxell A/S (formed as a spinout from Pharmexa—formally M&E Biotech) by entering into a global patent settlement concerning certain drug target identification technologies, which includes both cross-licensing and joint ownership to certain patents and allows for worldwide freedom of operation for both companies.
In September 2000, we entered into a Technology Transfer Agreement with Questcor Pharmaceuticals, Inc. and acquired the license and technology to a hepatitis C research program. Under the terms of this agreement, we have paid a nonrefundable and noncreditable fee of $500,000, issued to Questcor 83,333 shares of Series E preferred stock that subsequently converted to 83,333 shares of common stock upon completion of the our initial public offering and will be responsible for satisfying certain milestones and royalties. We were also committed to invest a total of $2.0 million in research and development expenses over a two-year period through 2002. This committed spending level was achieved midway through 2002. The agreement terminates upon the expiration of the last patent within the agreement. We accounted for the Series E preferred stock at $9.00 per share based on the deemed fair value of our common stock at the date of sale, and we expensed the aggregate value of approximately $1.2 million in September 2000, as the acquired technology was not yet fully developed and had no alternative use.
Critical Accounting Policies and the Use of Estimates
Our discussion and analysis of our financial condition and results of operations are based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates, including those related to terms of the research collaborations, investments, stock compensation, impairment issues, the estimated useful life of assets, and contingencies. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. We believe the following critical
30
accounting policies affect our more significant judgments and estimates used in the preparation of our financial statements.
Revenue Recognition
Non-refundable, up-front payments received in connection with research and development collaboration agreements, including technology access fees, are deferred and recognized on a straight-line basis over the relevant periods of continuing involvement, generally the research term.
Revenues related to collaborative research with our corporate collaborators are recognized as research services are performed over the related funding periods for each contract. Under these agreements, we are required to perform research and development activities as specified in each respective agreement. The payments received are not refundable and are generally based on a contractual cost per full-time equivalent employee working on the project. Research and development expenses under the collaborative research agreements approximate or exceed the revenue recognized under such agreements over the term of the respective agreements. Deferred revenue may result if we were not to incur the required level of effort during a specific period in comparison to funds received under the respective contracts.
Milestones are recognized pursuant to collaborative agreements upon the achievement of these specified at risk milestones.
Royalties will be recognized as earned in accordance with the contract terms when the third party results are reliably measurable and collectibility is reasonably assured.
Stock-based Compensation
We recorded no deferred stock compensation with respect to options granted to employees for the year ended December 31, 2002. We recorded deferred stock compensation with respect to options granted to employees of approximately $0.3 million and $4.9 million in the years ended December 31, 2001 and 2000, respectively, representing the difference between the deemed fair value of our common stock for financial reporting purposes on the date these options were granted and the exercise price. These amounts have been reflected as components of stockholders' equity, and the deferred expense is being amortized to operations over the vesting period of the options, generally four to five years, using the graded vesting method. We amortized deferred stock compensation of $1.0 million, $2.6 million and $4.9 million for the years ended December 31, 2002, 2001 and 2000, respectively. At December 31, 2002, we had a total of $0.8 million remaining to be amortized over the remaining vesting periods of the stock options.
In addition to the amortization of the deferred stock compensation, we also record charges associated with options granted to consultants in accordance with accounting principles generally accepted in the United States that involve the periodic revaluation of outstanding unvested consultant options based upon the current market value of our common stock and other assumptions, including the expected future volatility of our stock price. We recognized stock-based compensation recovery for revaluation of consultant options of $0.2 million and $0.5 million for the years ended December 31, 2002 and 2001, respectively. We recognized stock-based compensation expense for revaluation of consultant options of $5.3 million for the year ended December 31, 2000. Even though the number of unvested outstanding options issued to consultants continues to decline, we expect to see continued fluctuations in the future as a portion of these options are revalued based on the current market price of our common stock through the application of the graded vesting method.
31
Years Ended December 31, 2002, 2001 and 2000
Revenues. Contract revenues from collaborations were $15.8 million in 2002, compared to $15.3 million in 2001 and $13.2 million in 2000. Revenues in 2002, 2001 and 2000 consisted primarily of research support and amortization of upfront fees from the continuation of our collaborations with Pfizer, Johnson & Johnson, Novartis, and, in 2002 only, Daiichi. In 2002 and 2001, revenues also included milestone payments for targets delivered and accepted from certain collaborators. Revenue was flat in 2002 as compared to 2001 primarily due to a combination of the end of the research phase of the Pfizer collaboration, offset by a full year of the angiogenesis program with Novartis and the commencement of the collaboration with Daiichi. The increase in revenues of $2.1 million from 2000 to 2001 was primarily due to the commencement of the angiogenesis program with Novartis in July 2001 and milestones achieved in the Johnson & Johnson and Pfizer programs. We expect contract revenues from collaborations to be a significant component of our total revenues for the foreseeable future.
Research and Development. Research and development expenses were $43.4 million in 2002, compared to $32.3 million in 2001 and $32.0 million in 2000. Excluding stock-based compensation, research and development expenses were $42.8 million in 2002, compared to $30.7 million in 2001 and $22.9 million in 2000. The increase in 2002 of $12.1 million reflects primarily the continued expansion of our drug development infrastructure, the addition of both drug development and research headcount, increased outside contract efforts, increased preclinical activities, the commencement of clinical trials and costs associated with our intellectual property. In September 2002, we began the Phase I clinical trial of our lead compound, R112, in the United Kingdom and subsequently filed an IND application for this compound with the FDA for the clinical indication of allergic rhinitis. The increase in 2001 of $7.8 million primarily reflected the expansion of our drug development infrastructure, the addition of both drug development and research headcount, increased outside contract efforts, increased preclinical activities and costs associated with our intellectual property. We expect research and development expenses to increase in future years, particularly as we continue to move our solely-owned drug candidates through preclinical activities and into clinical trials.
The scope and magnitude of future research and development expenses are difficult to predict at this time given the number of studies that will need to be conducted for any of our potential products as well as our limited capital resources. In general, biopharmaceutical-development involves a series of steps—beginning with identification of a potential target and including, among others, proof of concept in animals and Phase I, II and III clinical studies in humans—each of which is typically more expensive than the previous step. Success in development therefore results in increasing expenditures. Our research and development expenditures currently include costs for scientific personnel, supplies, equipment, consultants, patent filings, sponsored research, allocated facility costs and costs related to clinical trials.
Because of the number of research projects we have ongoing at any one time, and the ability to utilize resources across several projects, the majority of our research and development costs are not directly tied to any individual project and are allocated among multiple projects. Our project management is based primarily on scientific data and supplemented by these cost allocations, which are based primarily on human resource time incurred on each project. As a result the costs allocated to a project do not necessarily reflect the actual costs of the project. Accordingly, we do not maintain actual cost incurred information for our projects on a project-by-project basis.
General and Administrative Expenses. General and administrative expenses were $9.5 million in 2002, compared to $8.0 million in 2001 and $6.7 million in 2000. The increases in both 2002 and 2001 of $1.5 million and $1.3 million, respectively, were primarily attributable to higher employee costs and greater infrastructure costs to support the growing research and development activities. We expect that general and administrative expenses will increase in the future to support the continued growth of our research and development efforts as our products continue to move into clinical trials.
32
Net Interest Expense. Net interest expense was $14,000 in 2002, compared with net interest income of $1.2 million in 2001 and $0.1 million in 2000. Interest income results from our interest-bearing cash and investment balances, whereas interest expense is the result of our capital lease obligations associated with fixed asset purchases. In 2002, interest expense exceeded interest income due primarily to a reduction in interest rates on our owned securities. The increase in net interest income in 2001 is directly related to the investment interest earned from the proceeds of our initial public offering in December of 2000.
Deemed Dividend to Series E Preferred Stockholders. In February 2000, we completed a private placement of 2,508,330 shares of series E preferred stock at $6.00 per share for net proceeds of approximately $15.1 million. At the date of issuance, we believed the per share price of $6.00 represented the fair value of the preferred stock. Subsequent to the commencement of the our initial public offering process, we re-evaluated the fair value of our common stock as of February 2000 and determined it to be $9.00 per share. Accordingly, the increase in fair value resulted in a beneficial conversion feature of $10.0 million that was recorded as a deemed dividend to the preferred stockholders in 2000. In August 2000, we issued 33,333 shares of series E preferred stock to one of our directors. We recorded a deemed dividend of approximately $100,000 at the time of issuance.
Effect of New Accounting Standards
In June 2002, the Financial Accounting Standards Board (or FASB) issued FAS 146, "Accounting for Costs Associated with Exit or Disposal Activities," which addresses accounting for restructuring, discontinued operation, plant closing or other exit or disposal activity. FAS 146 requires companies to recognize costs associated with exit or disposal activities when they are incurred rather than at the date of a commitment to an exit or disposal plan. FAS 146 is to be applied prospectively to exit or disposal activities initiated after December 31, 2002. The adoption of FAS 146 is not expected to have a significant impact on our financial position or results of operations.
In November 2002, the FASB issued Interpretation No. 45 (or FIN 45), "Guarantor's Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others." FIN 45 elaborates on the existing disclosure requirements for most guarantees, including residual value guarantees issued in conjunction with operating lease agreements. It also clarifies that at the time a company issues a guarantee, the company must recognize an initial liability for the fair value of the obligation it assumes under that guarantee and must disclose that information in its interim and annual financial statements. The initial recognition and measurement provisions apply on a prospective basis to guarantees issued or modified after December 31, 2002. We have made the disclosure requirements in our 2002. Our adoption of the recognition requirements of FIN 45 are not expected to have a material impact on our results of operations and financial position.
In January 2003, the FASB issued Interpretation No. 46 (or FIN 46), "Consolidation of Variable Interest Entities." FIN 46 requires a variable interest entity to be consolidated by a company if that company is subject to a majority of the risk of loss from the variable interest entity's activities or entitled to receive a majority of the entity's residual returns or both. A variable interest entity is a corporation, partnership, trust, or any other legal structures used for business purposes that either (a) does not have equity investors with voting rights or (b) has equity investors that do not provide sufficient financial resources for the entity to support its activities. A variable interest entity often holds financial assets, including loans or receivables, real estate or other property. A variable interest entity may be essentially passive or it may engage in research and development or other activities on behalf of another company. The consolidation requirements of FIN 46 apply immediately to variable interest entities created after January 31, 2003. The consolidation requirements apply to older entities in the first fiscal year or interim period beginning after June 15, 2003. Certain of the disclosure requirements apply to all financial statements issued after January 31, 2003, regardless of when the variable interest
33
entity was established. Our adoption of FIN 46 is not expected to have a material impact on our results of operations and financial position.
In December 2002, the FASB issued Statement No. 148, "Accounting for Stock-Based Compensation—Transition and Disclosure." FAS 148 amends FAS 123 "Accounting for Stock-Based Compensation" to provide alternative methods of transition for a voluntary change to the fair value based method of accounting for stock-based employee compensation. In addition, FAS 148 amends the disclosure requirements of FAS 123 to require more prominent disclosures in both annual and interim financial statements about the method of accounting for stock-based employee compensation and the effect of the method used on reported results. The additional disclosure requirements of FAS 148 are effective for fiscal years ending after December 15, 2002. We have elected to continue to follow the intrinsic value method of accounting as prescribed by Accounting Principles Board Opinion No. 25 (or APB 25), "Accounting for Stock Issued to Employees," to account for employee stock options.
In November 2002, the Emerging Issues Task Force (or EITF) reached a consensus on Issue No. 00-21, "Revenue Arrangements with Multiple Deliverables." EITF Issue No. 00-21 provides guidance on how to account for arrangements that involve the delivery or performance of multiple products, services and/or rights to use assets. The provisions of EITF Issue No. 00-21 will apply to revenue arrangements entered into in fiscal periods beginning after June 15, 2002. We believe the adoption of this standard will have no material impact on our financial statements.
Liquidity and Capital Resources
We have financed our operations from inception primarily through sales of equity securities, contract payments payable to us under our collaboration agreements and equipment financing arrangements. As of December 31, 2002, we had received $126.1 million in gross proceeds from the sale of equity securities, including $20.0 million from collaborators, and had received $57.8 million in research funding from collaborators. In addition, as of December 31, 2002, we had financed, through leases and loans, the purchase of equipment and leasehold improvements totaling approximately $17.2 million.
As of December 31, 2002, we had $27.3 million in cash, cash equivalents and available-for-sale securities, as compared to $33.4 million as of December 31, 2001, a decrease of $6.1 million. This decrease was attributable to a combination of approximately $34.5 million in net cash used in operating activities offset by proceeds of $31.2 million, net of commissions and offering costs, from the sale of 7,465,117 shares of our common stock in two offerings in January and February 2002 under our shelf registration statement. We also invested $1.6 million in capital equipment and had debt service payments of $3.7 million in conjunction with our equipment financing arrangements. These payments were offset by $2.0 million of proceeds from lease financing and $0.4 million from the sale of our stock through incentive stock option plans.
As of December 31, 2002, we had $5.7 million in capital lease obligations associated with our financed purchase of equipment and leasehold improvements. All existing equipment financing agreements as of December 31, 2002 are secured by the equipment financed, bear interest rates in a range of 7% to 15% and are due in monthly installments through 2005. In addition, three of these agreements have balloon payments at the end of each loan term, while the fourth agreement allows us to purchase the assets financed at the fair market value or 20% of the original acquisition cost at the end of the financing term. In July 2002, we entered into a tenant improvement and equipment lease line agreement for an aggregate total of $15.0 million. Due to the amendment of the master lease agreement for our 1180 Veterans Blvd. facility signed in October 2002 we have terminated this financing arrangement As of December 31, 2002, we had a total of $2.0 million available for draw down under all financing agreements.
34
During 2002 our office and research facility located at 240 East Grand in South San Francisco was leased under an operating lease that terminated in conjunction with a 15-year lease for our current office and research facilities at 1180 Veterans Blvd. in South San Francisco signed in May 2001. Under the terms of the lease signed in 2001, we were to occupy our new facilities in late 2002 and were to concurrently terminate our lease of our former facility at 240 East Grand in South San Francisco. We determined that the 2001 lease for our current facility was an operating lease in accordance with FAS 13. In connection with the termination of the current 240 East Grand lease, we accelerated the amortization of tenant improvements and accrued rent charges over the expected remaining life of the lease and incurred minimal costs in connection with the terminated lease. The 1180 Veterans Blvd. research and office facilities were constructed as a build-to-suit facility. Under the original lease, we were obligated to fund approximately $18.0 million of the total tenant improvement obligations. In October 2002, we amended this original lease to provide for a delay of the rent commencement date until February 1, 2003 and an increase in the tenant improvement allowance to cover all of the expected remaining construction obligations on the facility. The lease was also amended to increase the future rental commitments to compensate for the delay of the rent commencement and the increase in the tenant improvement allowance. Since the amendment was considered a material change to the original lease, we reviewed the accounting treatment for this amended lease and again determined the lease to be an operating lease. We moved into the new facility during February 2003.
Prior to the signing of the amendment, we had been directly paying a portion of the pre-construction and construction costs related to the new facility. These costs were being capitalized on our balance sheet as construction-in progress. Per the terms of the amendment, we have estimated that the landlord will be responsible for reimbursing to us all of the costs that we had previously capitalized. Therefore, we have reclassified these costs into a short-term asset "Receivable from Landlord" in our financial statements.
The following are our contractual commitments (by fiscal year) as of December 31, 2002 associated with debt obligations, lease obligations, and contracted research obligations:
| |
Total |
2003 |
2004 - 2005 |
2006 - 2007 |
2008 - 2018 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(in thousands) |
|||||||||||||||
| Capital leases | $ | 6,322 | $ | 3,815 | $ | 2,507 | $ | — | $ | — | ||||||
| Facilities leases | 198,128 | 7,169 | 21,438 | 26,546 | 142,975 | |||||||||||
| Contracted research | 500 | 500 | — | — | — | |||||||||||
| Total | $ | 204,950 | $ | 11,484 | $ | 23,945 | $ | 26,546 | $ | 142,975 | ||||||
On January 31, 2003, we implemented a restructuring plan to reduce the rate of our cash consumption and better align our operating structure with current and expected future economic conditions. The restructuring plan included an immediate reduction in force of approximately 16 percent, or 25 employees, to 135 employees with reductions occurring in all functional areas. Two of our officers were included in this reduction in force. We also deferred a portion of certain officers' salaries.
We believe that our existing capital resources, together with anticipated payments under current collaborations, will be sufficient to support our current operating plan and spending through the end of September 2003. We will require additional financing to fund our operations as currently planned beyond that date. While we have been actively seeking both financing and corporate partnering opportunities, we cannot assure you that a sufficient financing or corporate partnering transaction can be completed on acceptable terms, or at all. If a sufficient financing or corporate partnering transaction cannot be completed or assured, we will not be able to continue our current operating plans and will be forced to reduce the scale of our operations. If a sufficient financing or corporate partnering transaction is not reasonably assured by the middle of May 2003, we will complete our R112 clinical trial currently under way and continue only with certain external preclinical studies in our Hepatitis C
35
program. All other external studies would be terminated. If as of June 30, 2003 a sufficient financing or corporate partnering transaction is not reasonably assured, we will be required to significantly scale back our operations by reducing our headcount by approximately 50% and significantly reducing all discretionary spending. We anticipate that upon the execution of these actions, our existing capital resources will be sufficient to support the substantially reduced funding of our current programs as well as our operations through the end of 2003. To the extent we raise additional capital by issuing equity securities, our stockholders would at this time experience substantial dilution.
Our future funding requirements will depend on many factors, including, but not limited to:
In addition, we are constantly reviewing potential opportunities to expand our technologies or add to our portfolio of drug candidates. In the future, we may need further capital in order to acquire or invest in technologies, products or businesses. For the next several years, we do not expect the cash generated from our operations to generate the amount of cash required by our future cash needs. We expect to finance future cash needs through strategic collaborations, debt financing and the sale of equity securities. We cannot assure you that additional financing or collaboration and licensing arrangements will be available when needed or that, if available, this financing will be obtained on terms favorable to us or our stockholders. Insufficient funds may require us to delay, scale back or eliminate some or all of our research or development programs, to lose rights under existing licenses or to relinquish greater or all rights to product candidates at an earlier stage of development or on less favorable terms than we would otherwise choose or may adversely affect our ability to operate as a going concern. If additional funds are obtained by issuing equity securities, substantial dilution to existing stockholders may result.
As of December 31, 2002, we had federal net operating loss carryforwards of approximately $90.0 million to offset future taxable income. We also had federal research and development tax credit carryforwards of approximately $3.4 million. If not utilized, net operating loss and credit carryforwards will begin to expire in 2011. Utilization of the net operating losses and credits may be subject to a substantial annual limitation due to ownership change limitations provided by the Internal Revenue
36
Code of 1986. The annual limitation may result in the expiration of our net operating losses and credits before they can be used. You should read Note 8 of the notes to our financial statements.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
The primary objective of our investment activities is to preserve principal while at the same time maximizing the income we receive from our investments without significantly increasing risk. Some of the securities in which we invest may have market risk. This means that a change in prevailing interest rates may cause the fair value amount of the investment to fluctuate. For example, if we hold a security that was issued with a fixed interest rate at the then-prevailing rate and the prevailing interest rate later rises, the market value amount of our investment will decline. To minimize this risk in the future, we intend to maintain our portfolio of cash equivalents and short-term investments in a variety of securities, including commercial paper, money market funds and government and non-government debt securities. In 2002, 2001 and 2000, we maintained an investment portfolio primarily in depository accounts and corporate commercial paper. Due to the short-term nature of these investments, we believe we do not have a material exposure to interest rate risk arising from our investments. Therefore, no quantitative tabular disclosure is provided.
We have operated primarily in the United States, and all funding activities with our collaborators to date have been made in U.S. dollars. Accordingly, we have not had any exposure to foreign currency rate fluctuations.
37
Item 8. Financial Statements and Supplementary Data
INDEX TO FINANCIAL STATEMENTS
Rigel Pharmaceuticals, Inc.
| |
Page |
|
|---|---|---|
| Report of Ernst & Young LLP, Independent Auditors | 39 | |
Balance Sheets |
40 |
|
Statement of Operations |
41 |
|
Statement of Stockholders' Equity |
42 |
|
Statements of Cash Flows |
43 |
|
Notes to Financial Statements |
44 |
38
Report of Ernst & Young LLP, Independent Auditors
The
Board of Directors and Stockholders
Rigel Pharmaceuticals, Inc.
We have audited the accompanying balance sheets of Rigel Pharmaceuticals, Inc. as of December 31, 2002 and 2001, and the related statements of operations, stockholders' equity, and cash flows for each of the three years in the period ended December 31, 2002. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Rigel Pharmaceuticals, Inc. at December 31, 2002 and 2001, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2002, in conformity with accounting principles generally accepted in the United States.
| /s/ ERNST & YOUNG LLP |
Palo
Alto, California
January 24, 2003 except for Note 9 as to
which the date is January 31, 2003.
39
RIGEL PHARMACEUTICALS, INC.
BALANCE SHEETS
(In thousands, except share and per share amounts)
| |
December 31, |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
2002 |
2001 |
|||||||
| Assets | |||||||||
| Current assets: | |||||||||
| Cash and cash equivalents | $ | 26,535 | $ | 11,488 | |||||
| Available-for-sale securities | 756 | 21,927 | |||||||
| Accounts receivable | 1,503 | 1,153 | |||||||
| Receivable from landlord | 6,175 | 389 | |||||||
| Prepaid expenses and other current assets | 1,894 | 1,576 | |||||||
| Total current assets | 36,863 | 36,533 | |||||||
Property and equipment, net |
5,206 |
8,440 |
|||||||
| Other assets | 2,273 | 1,475 | |||||||
| $ | 44,342 | $ | 46,448 | ||||||
Liabilities and stockholders' equity |
|||||||||
| Current liabilities: | |||||||||
| Accounts payable | $ | 3,460 | $ | 1,952 | |||||
| Accrued compensation | 799 | 671 | |||||||
| Accrued liabilities | 2,662 | 1,104 | |||||||
| Deferred revenue | 4,061 | 3,264 | |||||||
| Capital lease obligations | 3,388 | 3,171 | |||||||
| Total current liabilities | 14,370 | 10,162 | |||||||
Capital lease obligations |
2,313 |
4,243 |
|||||||
| Long-term portion of deferred revenue | 2,147 | 2,240 | |||||||
| Other long-term liabilities | 71 | 862 | |||||||
Commitments |
|||||||||
Stockholders' equity: |
|||||||||
| Common stock, $0.001 par value; 100,000,000 shares authorized; 45,702,227 and 37,732,209 shares issued and outstanding in 2002 and 2001, respectively | 46 | 38 | |||||||
| Additional paid-in capital | 140,982 | 109,095 | |||||||
| Deferred stock compensation | (772 | ) | (2,452 | ) | |||||
| Accumulated other comprehensive (loss) income | (1 | ) | 44 | ||||||
| Accumulated deficit | (114,814 | ) | (77,784 | ) | |||||
| Total stockholders' equity | 25,441 | 28,941 | |||||||
| $ | 44,342 | $ | 46,448 | ||||||
See accompanying notes.
40
RIGEL PHARMACEUTICALS, INC.
STATEMENT OF OPERATIONS
(In thousands, except per share amounts)
| |
Years ended December 31, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2002 |
2001 |
2000 |
||||||||
| Contract revenues from collaborations | $ | 15,788 | $ | 15,303 | $ | 13,218 | |||||
| Costs and expenses: | |||||||||||
| Research and development (See Note A) | 43,350 | 32,313 | 32,034 | ||||||||
| General and administrative (See Note A) | 9,454 | 7,950 | 6,689 | ||||||||
| 52,804 | 40,263 | 38,723 | |||||||||
| Loss from operations | (37,016 | ) | (24,960 | ) | (25,505 | ) | |||||
| Interest income | 856 | 1,957 | 1,078 | ||||||||
| Interest expense | (870 | ) | (802 | ) | (933 | ) | |||||
| Net loss | (37,030 | ) | (23,805 | ) | (25,360 | ) | |||||
| Deemed dividend to Series E preferred stockholders | — | — | (10,133 | ) | |||||||
| Net loss allocable to common stockholders | $ | (37,030 | ) | $ | (23,805 | ) | $ | (35,493 | ) | ||
| Net loss per common share, basic and diluted | $ | (0.82 | ) | $ | (0.64 | ) | $ | (4.89 | ) | ||
| Weighted average shares used in computing net loss per common share, basic and diluted | 44,954 | 37,287 | 7,263 | ||||||||
Note A: |
|||||||||||
| Includes charges for stock-based compensation as follows: | |||||||||||
Research and development |
$ |
568 |
$ |
1,596 |
$ |
9,184 |
|||||
| General and administrative | 191 | 527 | 976 | ||||||||
| $ | 759 | $ | 2,123 | $ | 10,160 | ||||||
See accompanying notes.
41
RIGEL PHARMACEUTICALS, INC.
STATEMENT OF STOCKHOLDERS' EQUITY
(In thousands, except per share and per share amounts)
| |
|
|
Common Stock |
|
|
Accumulated Other Comprehensive Income |
|
Total Stock- holders Equity |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Convertible Shares |
Preferred Stock Amount |
Additional Paid-in Capital |
Deferred Stock Compensation |
Accumulated Deficit |
|||||||||||||||||||||
| |
Shares |
Amount |
||||||||||||||||||||||||
| Balance at December 31, 1999 | 22,053,887 | $ | 22 | 3,095,834 | $ | 3 | $ | 35,164 | $ | (5,814 | ) | $ | — | $ | (28,619 | ) | $ | 756 | ||||||||
| Issuance of Series E preferred stock at $6.00 per share for cash, net of issuance cost | 2,541,663 | 3 | — | — | 15,247 | — | — | — | 15,250 | |||||||||||||||||
| Issuance of Series E preferred stock in exchange for a technology license | 133,333 | — | — | — | 1,250 | — | — | — | 1,250 | |||||||||||||||||
| Issuance of Series D preferred stock upon exercise of warrant at $2.00 per share | 167,074 | — | — | — | 215 | — | — | — | 215 | |||||||||||||||||
| Conversion of preferred stock to common stock upon closing of initial public offering | (24,895,957 | ) | (25 | ) | 24,895,957 | 25 | — | — | — | — | — | |||||||||||||||
| Issuance of common stock at $7.00 per share for cash, net of issuance costs | — | — | 7,078,571 | 7 | 45,553 | — | — | — | 45,560 | |||||||||||||||||
| Issuance of common stock upon exercise of options | — | — | 1,633,824 | 2 | 275 | — | — | — | 277 | |||||||||||||||||
| Issuance of common stock for services | — | — | 100,000 | — | 900 | — | — | — | 900 | |||||||||||||||||
| Compensation expense related to options granted to consultants | — | — | — | — | 5,280 | — | — | — | 5,280 | |||||||||||||||||
| Deferred stock compensation | — | — | — | — | 4,858 | (4,858 | ) | — | — | — | ||||||||||||||||
| Amortization of deferred stock compensation | — | — | — | — | — | 4,880 | — | — | 4,880 | |||||||||||||||||
| Net loss and comprehensive loss | — | — | — | — | — | — | 2 | (25,360 | ) | (25,358 | ) | |||||||||||||||
| Balance at December 31, 2000 | — | — | 36,804,186 | 37 | 108,742 | (5,792 | ) | 2 | (53,979 | ) | 49,010 | |||||||||||||||
| Issuance of common stock upon exercise of options and participation in Purchase Plan | — | — | 928,023 | 1 | 887 | — | — | — | 888 | |||||||||||||||||
| Issuance of warrant to purchase common stock for services | — | — | — | — | 683 | — | — | — | 683 | |||||||||||||||||
| Compensation recovery related to options granted to consultants | — | — | — | — | (510 | ) | — | — | — | (510 | ) | |||||||||||||||
| Deferred stock compensation | — | — | — | — | 285 | (285 | ) | — | — | — | ||||||||||||||||
| Amortization of deferred stock compensation, net of cancellations | — | — | — | — | (992 | ) | 3,625 | — | — | 2,633 | ||||||||||||||||
| Net loss and comprehensive loss | — | — | — | — | — | — | 42 | (23,805 | ) | (23,763 | ) | |||||||||||||||
| Balance at December 31, 2001 | — | — | 37,732,209 | 38 | 109,095 | (2,452 | ) | 44 | (77,784 | ) | 28,941 | |||||||||||||||
| Issuance of common stock at $4.50 per share for cash, net of issuance costs | — | — | 7,000,000 | 7 | 29,421 | — | — | — | 29,428 | |||||||||||||||||
| Issuance of common stock at $4.30 per share for cash, net of issuance costs | — | — | 465,117 | — | 1,923 | — | — | — | 1,923 | |||||||||||||||||
| Issuance of common stock upon exercise of options and participation in Purchase Plan | — | — | 504,901 | 1 | 445 | — | — | — | 446 | |||||||||||||||||
| Issuance of warrants to purchase common stock for services | — | — | — | — | 1,018 | — | — | — | 1,018 | |||||||||||||||||
| Compensation recovery related to options granted to consultants | — | — | — | — | (196 | ) | — | — | — | (196 | ) | |||||||||||||||
| Amortization of deferred stock compensation, net of cancellations | — | — | — | — | (724 | ) | 1,680 | — | — | 956 | ||||||||||||||||
| Net loss and comprehensive loss | — | — | — | — | — | — | (45 | ) | (37,030 | ) | (37,075 | ) | ||||||||||||||
| Balance at December 31, 2002 | — | $ | — | 45,702,227 | $ | 46 | $ | 140,982 | $ | (772 | ) | $ | (1 | ) | $ | (114,814 | ) | $ | 25,441 | |||||||
See accompanying notes
42
RIGEL PHARMACEUTICALS, INC.
STATEMENTS OF CASH FLOWS
(In thousands)
| |
Years ended December 31, |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2002 |
2001 |
2000 |
||||||||||
| Operating activities | |||||||||||||
| Net loss | $ | (37,030 | ) | $ | (23,805 | ) | $ | (25,360 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||||
| Depreciation and amortization | 4,868 | 4,127 | 2,677 | ||||||||||
| Amortization of deferred stock compensation, net | 956 | 2,633 | 4,880 | ||||||||||
| Noncash stock (recovery) compensation | (196 | ) | (510 | ) | 5,280 | ||||||||
| Issuances of equity instruments for noncash benefits | 20 | — | 2,150 | ||||||||||
| Changes in assets and liabilities: | |||||||||||||
| Accounts receivable | (350 | ) | (490 | ) | 1,685 | ||||||||
| Prepaid expenses and other current assets, including receivable from landlord | (4,593 | ) | (939 | ) | (680 | ) | |||||||
| Other assets | 201 | (551 | ) | — | |||||||||
| Accounts payable | 1,480 | 638 | 431 | ||||||||||
| Accrued compensation | 128 | (53 | ) | 436 | |||||||||
| Accrued liabilities | 74 | 408 | (707 | ) | |||||||||
| Deferred revenue | 704 | 2,734 | (2,956 | ) | |||||||||
| Other long-term liabilities | (790 | ) | (173 | ) | 576 | ||||||||
| Net cash used in operating activities | (34,528 | ) | (15,981 | ) | (11,588 | ) | |||||||
Investing activities |
|||||||||||||
| Purchases of available-for-sale securities | (26,713 | ) | (47,511 | ) | (3,962 | ) | |||||||
| Maturities of available-for-sale securities | 22,875 | 29,590 | — | ||||||||||
| Sales of available-for-sale securities | 24,964 | — | — | ||||||||||
| Capital expenditures | (1,635 | ) | (3,229 | ) | (3,617 | ) | |||||||
| Net cash provided (used) in investing activities | 19,491 | (21,150 | ) | (7,579 | ) | ||||||||
| Financing activities | |||||||||||||
| Proceeds from capital lease financing | 1,999 | 1,748 | 3,471 | ||||||||||
| Principal payments on capital lease obligations | (3,712 | ) | (3,047 | ) | (2,412 | ) | |||||||
| Net proceeds from issuances of common stock | 31,797 | 888 | 45,837 | ||||||||||
| Net proceeds from issuances of convertible preferred stock | — | — | 15,465 | ||||||||||
| Net cash provided by (used in) financing activities | 30,084 | (411 | ) | 62,361 | |||||||||
| Net increase (decrease) in cash and cash equivalents | 15,047 | (37,542 | ) | 43,194 | |||||||||
| Cash and cash equivalents at beginning of period | 11,488 | 49,030 | 5,836 | ||||||||||
| Cash and cash equivalents at end of period | $ | 26,535 | $ | 11,488 | $ | 49,030 | |||||||
| Supplemental disclosure of cash flow information | |||||||||||||
| Interest paid | $ | 870 | $ | 802 | $ | 933 | |||||||
| Schedule of non cash transactions | |||||||||||||
| Deferred stock compensation | $ | — | $ | 285 | $ | 4,858 | |||||||
| Issuance of warrants for services | $ | 1,018 | $ | 683 | $ | — | |||||||
| Series E deemed dividend | $ | — | $ | — | $ | 10,133 | |||||||
See accompanying notes.
43
Rigel Pharmaceuticals, Inc.
NOTES TO FINANCIAL STATEMENTS
In this Annual Report, "Rigel," "we," "us" and "our" refer to Rigel Pharmaceuticals, Inc. "Common Stock" refers to Rigel's common stock, par value $0.001 per share.
1. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Nature of operations and basis of presentation
We were incorporated in the state of Delaware on June 14, 1996. We are engaged in the discovery and development of a broad range of new small molecule drug candidates.
Management's Plans
We have incurred net losses since inception and expect to incur substantial and increasing losses for the next several years as we continue to move drug candidates into and through preclinical and clinical stages of drug development and expand our research and development activities. To date, we have funded our operations primarily through the sale of equity securities, non-equity payments from collaborative partners and capital asset lease financings. We believe that our existing capital resources, together with anticipated payments under current collaborations, will be sufficient to support our current operating plan and spending through the end of September 2003. We will require additional financing to fund our operations as currently planned beyond that date. While we have been actively seeking both financing and corporate partnering opportunities, we cannot assure you that a sufficient financing or corporate partnering transaction can be completed on acceptable terms, or at all. If a sufficient financing or corporate partnering transaction cannot be completed or assured, we will not be able to continue our current operating plans and will be forced to reduce the scale of our operations. If a sufficient financing or corporate partnering transaction is not reasonably assured by the middle of May 2003, we will complete our R112 clinical trial currently under way and continue only with certain external preclinical studies in our Hepatitis C program. All other external studies would be terminated. If as of June 30, 2003 a sufficient financing or corporate partnering transaction is not reasonably assured, we will be required to significantly scale back our operations by reducing our headcount by approximately 50% and significantly reducing all discretionary spending. We anticipate that upon the execution of these actions, our existing capital resources will be sufficient to support the substantially reduced funding of our current programs as well as our operations through the end of 2003. To the extent we raise additional capital by issuing equity securities, our stockholders would at this time experience substantial dilution.
Use of estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from these estimates.
Stock Award Plans
We have elected to continue to follow Accounting Principles Board Opinion No. 25 (or APB 25), "Accounting for Stock Issued to Employees," to account for employee stock options because the alternative fair value method of accounting prescribed by Statement of Financial Accounting Standards (or FAS) No. 123, "Accounting for Stock-Based Compensation," requires the use of option valuation models that were not developed for use in valuing employee stock options. Under APB 25, the intrinsic
44
value method of accounting, no compensation expense is recognized because the exercise price of our employee stock options equals the market price of the underlying stock on the date of grant.
In December 2002, the FASB issued Statement No. 148, "Accounting for Stock-Based Compensation—Transition and Disclosure." FAS 148 amends FAS 123 "Accounting for Stock-Based Compensation" to provide alternative methods of transition for a voluntary change to the fair value based method of accounting for stock-based employee compensation. In addition, FAS 148 amends the disclosure requirements of FAS 123 to require more prominent disclosures in both annual and interim financial statements about the method of accounting for stock-based employee compensation and the effect of the method used on reported results. The additional disclosure requirements of FAS 148 are effective for fiscal years ending after December 15, 2002. We have elected to continue to follow the intrinsic value method of accounting as prescribed by Accounting Principles Board Opinion No. 25 (or APB 25), "Accounting for Stock Issued to Employees," to account for employee stock options. See Note 1 "Significant Accounting Polices" for the disclosures required by FAS 148.
Pro forma information regarding net loss and net loss per share is required by SFAS 123 and SFAS 148 and has been determined as if we had accounted for our employee stock options and employee stock purchase plan under the fair value method prescribed by the Statement. The fair value for these options was estimated at the date of grant using the Black-Scholes model in both 2002 and 2001, and the minimum value method in 2000 with the following weighted-average assumptions for the years ended December 31, 2002, 2001 and 2000: risk-free interest rates of 2.1%, 3.7% and 4.8%, respectively; volatility of 0.85 in 2002 and 0.65 in 2001and 2000; an expected option life of five years; and no dividend yield.
For purposes of pro forma disclosures, the estimated fair value of the options is amortized to expense over the vesting period of the options. Our pro forma information follows (in thousands, except per share amounts):
| |
Years Ended December 31, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2002 |
2001 |
2000 |
||||||||
| Net loss allocable to common stockholders—as reported: | $ | (37,030 | ) | $ | (23,805 | ) | $ | (35,493 | ) | ||
| Add: Total stock-based compensation determined under APB 25 | (759 | ) | (2,123 | ) | (10,160 | ) | |||||
| Add: Total stock-based compensation expense determined under the fair value based method for all awards | 4,150 | 4,931 | 11,976 | ||||||||
| Pro forma net loss | (40,421 | ) | (26,613 | ) | (37,309 | ) | |||||
| Basic and diluted net loss per common share: | |||||||||||
| As reported | $ | (0.82 | ) | $ | (0.64 | ) | $ | (4.89 | ) | ||
| Pro forma | (0.90 | ) | (0.71 | ) | (5.14 | ) | |||||
Cash, cash equivalents and available-for-sale securities
We consider all highly liquid investments in debt securities with a remaining maturity from the date of purchase of 90 days or less to be cash equivalents. Cash equivalents consist of money market funds and corporate debt securities. Our short-term investments include obligations of governmental agencies and corporate debt securities. By policy, we limit the concentration of credit risk by diversifying our investments among a variety of high credit-quality issuers.
45
All cash equivalents and short-term investments are classified as available-for-sale. Available-for-sale securities are carried at amortized cost, and approximated their fair value at December 31, 2002 and 2001. Unrealized gains (losses) are reported in stockholders' equity and included in other comprehensive income. Fair value is estimated based on available market information. The cost of securities sold is based on the specific identification method. For the years ended December 31, 2002, 2001 and 2000, gross realized gains and losses on available-for-sale securities were not material. See Note 4 for a summary of available-for-sale securities at December 31, 2002 and 2001.
Fair value of financial instruments
Financial instruments, including cash and cash equivalents, accounts receivable, accounts payable, accrued liabilities and accrued compensation are carried at cost or amortized cost, which management believes approximates fair value.
Derivative financial instruments and hedging activities
All derivatives are required to be recognized on the balance sheet at fair value. Derivatives that are not designated as hedges must be adjusted to fair value through earnings. If the derivative is designated and qualifies as a hedge, depending on the nature of the hedge, changes in the fair value of the derivative are either offset against the change in fair value of assets, liabilities, or firm commitments through earnings or recognized in other comprehensive income until the hedged item is recognized in earnings. The ineffective portion of a derivative's change in fair value will be immediately recognized in earnings. We do not hold derivative financial instruments and do no currently engage in hedging activities.
Property and equipment
Property and equipment are stated at cost. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets, which range from three to seven years. Leasehold improvements are amortized using the straight-line method over the estimated useful lives of the assets or the term of the lease, whichever is shorter.
Revenue recognition
Non-refundable, up-front payments received in connection with research and development collaboration agreements, including technology access fees, are deferred and recognized on a straight-line basis over the relevant periods of continuing involvement, generally the research term.
Revenues related to collaborative research with our corporate collaborators are recognized as research services are performed over the related development funding periods for each contract. Under these agreements, we are required to perform research and development activities as specified in each respective agreement. The payments received are not refundable and are generally based on a contractual cost per full-time equivalent employee working on the project. Research and development expenses under the collaborative research agreements approximate or exceed the revenue recognized under such agreements over the term of the respective agreements. Deferred revenue may result if we were not to incur the required level of effort during a specific period in comparison to funds received under the respective contracts.
Milestones are recognized pursuant to collaborative agreements upon the achievement of these specified at risk milestones.
46
Royalties will be recognized as earned in accordance with the contract terms when the third party results are reliably measurable and collectibility is reasonably assured.
Research and development
Research and development expenses include costs for scientific personnel, supplies, equipment, consultants, patent filings, research sponsored by us, allocated facility costs and costs related to clinical trials. All such costs are charged to research and development expense as incurred. Collaboration agreements generally specify minimum levels of research effort required to be performed by us.
Impairment of long-lived assets
We adopted FAS 144, "Accounting for the Impairment or Disposal of Long-Lived Assets," on January 1, 2002. FAS 144 supersedes FAS 121, "Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to Be Disposed Of." The primary objectives of FAS 144 are to develop one accounting model based on the framework established in FAS 121 for long-lived assets to be disposed of by sale, and to address significant implementation issues. Our adoption of FAS 144 did not have a material impact on our financial position or results of operations.
Segment reporting
We have determined that we operate in only one segment.
Contingencies
We are subject to claims related to the patent protection of certain of our technologies. We are required to assess the likelihood of any adverse judgments or outcomes to these matters as well as potential ranges of probable losses. A determination of the amount of reserves required, if any, for these contingencies is made after careful analysis of each individual issue. The required reserves may change in the future due to new developments in each matter or changes in approach such as a change in settlement strategy in dealing with these matters.
Net loss per share
Net loss per share has been computed according to Financial Accounting Standards Board Statement No. 128, "Earnings Per Share," which requires disclosure of basic and diluted earnings per share. Basic earnings per share excludes any dilutive effects of options, shares subject to repurchase, warrants and convertible securities. Diluted earnings per share includes the impact of potentially dilutive securities.
Our preferred stock converted into common stock upon the closing of our initial public offering in December 2000. For informational purposes, the following unaudited pro forma net loss per share data
47
reflects the assumed conversion of our preferred stock at the date of issuance (in thousands, except per share information):
| |
Year Ended December 31, 2000 |
|||
|---|---|---|---|---|
| Net loss to common stockholders before deemed dividend | $ | (25,360 | ) | |
| Weighted-average shares of common stock outstanding | 7,263 | |||
| Pro forma adjustment to reflect weighted average effect of assumed conversion of preferred stock | 22,280 | |||
| Total weighted average shares outstanding pro forma | 29,543 | |||
| Basic and diluted pro forma loss per share | $ | (0.86 | ) | |
During all periods presented, we had securities outstanding which could potentially dilute basic earnings per share in the future, but were excluded from the computation of diluted net loss per share, as their effect would have been antidilutive. These outstanding securities consist of the following (in thousands, except per share information):
| |
December 31, |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
2002 |
2001 |
2000 |
||||||
| Outstanding options | 6,465 | 5,761 | 5,700 | ||||||
| Warrants | 1,150 | 300 | 457 | ||||||
| Weighted average exercise price of options | $ | 3.47 | $ | 3.48 | $ | 2.70 | |||
| Weighted average exercise price of warrants | $ | 2.76 | $ | 5.03 | $ | 1.01 | |||
Recent accounting pronouncements
In June 2002, the Financial Accounting Standards Board (or FASB) issued FAS 146, "Accounting for Costs Associated with Exit or Disposal Activities," which addresses accounting for restructuring, discontinued operation, plant closing or other exit or disposal activity. FAS 146 requires companies to recognize costs associated with exit or disposal activities when they are incurred rather than at the date of a commitment to an exit or disposal plan. FAS 146 is to be applied prospectively to exit or disposal activities initiated after December 31, 2002. The adoption of FAS 146 is not expected to have a significant impact on our financial position and results of operations.
In November 2002, the FASB issued Interpretation No. 45 (or FIN 45), "Guarantor's Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others." FIN 45 elaborates on the existing disclosure requirements for most guarantees, including residual value guarantees issued in conjunction with operating lease agreements. It also clarifies that at the time a company issues a guarantee, the company must recognize an initial liability for the fair value of the obligation it assumes under that guarantee and must disclose that information in its interim and annual financial statements. The initial recognition and measurement provisions apply on a prospective basis to guarantees issued or modified after December 31, 2002. The disclosure requirements are effective for financial statements of interim or annual periods ending after December 15, 2002. Our adoption of FIN 45 did not have a material impact on our results of operations and financial position.
In January 2003, the FASB issued Interpretation No. 46 (or FIN 46), "Consolidation of Variable Interest Entities." FIN 46 requires a variable interest entity to be consolidated by a company if that company is subject to a majority of the risk of loss from the variable interest entity's activities or
48
entitled to receive a majority of the entity's residual returns or both. A variable interest entity is a corporation, partnership, trust, or any other legal structures used for business purposes that either (a) does not have equity investors with voting rights or (b) has equity investors that do not provide sufficient financial resources for the entity to support its activities. A variable interest entity often holds financial assets, including loans or receivables, real estate or other property. A variable interest entity may be essentially passive or it may engage in research and development or other activities on behalf of another company. The consolidation requirements of FIN 46 apply immediately to variable interest entities created after January 31, 2003. The consolidation requirements apply to older entities in the first fiscal year or interim period beginning after June 15, 2003. Certain of the disclosure requirements apply to all financial statements issued after January 31, 2003, regardless of when the variable interest entity was established. Our adoption of FIN 46 did not have a material impact on our results of operations and financial position.
In November 2002, the Emerging Issues Task Force (or EITF) reached a consensus on Issue No. 00-21, "Revenue Arrangements with Multiple Deliverables." EITF Issue No. 00-21 provides guidance on how to account for arrangements that involve the delivery or performance of multiple products, services and/or rights to use assets. The provisions of EITF Issue No. 00-21 will apply to revenue arrangements entered into in fiscal periods beginning after June 15, 2002. We believe the adoption of this standard will have no material impact on our financial statements.
2. SPONSORED RESEARCH AND LICENSE AGREEMENTS
Research agreements
On December 4, 1998, we entered into a research collaboration agreement with Johnson and Johnson Pharmaceutical and Development, LLC to research and identify novel targets for drug discovery. Under the terms of the contract, Johnson & Johnson paid a one-time non-refundable, non-creditable fee and will provide support for research activities during the research period, as well as various milestones and royalties. In December of 2001, Johnson & Johnson extended the funded research portion of the collaboration through December 2003. Johnson & Johnson participated in our series D and E preferred stock financings. Johnson & Johnson contributed $3,000,000 for 1,500,000 shares of series D preferred stock and contributed $1,000,000 for 166,666 shares of series E preferred stock. The preferred stock purchased by Johnson & Johnson automatically converted to 1,666,666 shares of common stock upon completion of our initial public offering.
On January 31, 1999, we entered into a two-year collaborative research agreement with Pfizer Inc. to discover and develop various molecular targets. Upon signing of the agreement, Pfizer was obligated to pay a one-time, nonrefundable, noncreditable fee. Under the terms of the contract, Pfizer provided support for research for two years and is obligated to pay us various milestones and royalties if certain conditions are met. On January 25, 2001, Pfizer notified us that it was electing to exercise its option to extend the funded research portion of the collaboration one additional year to January 31, 2002 and then extended it again for one additional month to February 28, 2002. In February 2002, the research phase of our collaboration with Pfizer concluded with Pfizer accepting a total of seven validated targets. Under our collaboration with Pfizer, we expect that these validated targets will continue through the drug discovery and development process at Pfizer. In conjunction with the original agreement, Pfizer contributed $2,000,000 in exchange for 1,000,000 shares of series D preferred stock that subsequently converted to 1,000,000 shares of common stock upon completion of the our initial public offering.
49
On May 28, 1999, we entered into a broad collaboration with Novartis Pharma AG, whereby we and Novartis agreed to work on up to five different research programs to identify various targets for drug development. Two programs were initiated in 1999 while the third program to be conducted at Novartis was initiated on January 1, 2000. In July 2001, we expanded our collaboration with Novartis with the initiation of our angiogenesis program, the fourth and final program in our Novartis collaboration. Pursuant to the expanded Novartis collaboration, we received a $4.0 million up-front payment from Novartis, which will be recognized as revenue ratably over the life of the contract. In addition, the expanded collaboration provides that the angiogenesis research program will be carried out at Rigel, provides for research reimbursement over the next three years and includes potential future milestones and royalty payments to Rigel. Novartis notified us that it has chosen not to exercise its option for a second program of research that would have been carried out at Novartis. In May 2002, Novartis elected to conclude the research phases of our two initial joint projects in the autoimmunity and transplant rejection areas, after 42 months each, effective in November 2002 and February 2003, respectively. Pursuant to the collaboration agreement, Novartis had the option to end the research phase on these programs after either 24 months or 42 months.
For all programs, Novartis will provide payment for various milestones and royalties if certain conditions in the collaboration agreement are met. In conjunction with the original agreement, Novartis contributed $4,000,000 in exchange for 2,000,000 shares of series D preferred stock that converted to 2,000,000 shares of common stock upon the completion of our initial public offering. The agreement also allowed for an additional equity investment of up to $10,000,000, which was callable by us up through an initial public offering. We exercised this right and sold to Novartis 1,428,571 shares of common stock at $7.00 per share concurrent with the closing of our initial public offering.
In August 2002, we signed an agreement for the establishment of collaboration with Daiichi Pharmaceuticals Co., Ltd to pursue research related to a specific protein degradation target. Per the agreement, the research phase of this collaboration is for three years. We will be working with Daiichi to discover and develop cancer pharmaceutical drugs. Under the terms of the collaboration agreement, Daiichi has paid us an upfront amount and a milestone payment, is obligated to pay us ongoing research support and may become obligated to pay us certain other milestones payments. In addition, we will receive royalties on any commercialized products to emerge from the collaboration.
The initial stages of the collaboration focused on the development of the assay for this specific target and the initiation of HTS to identify therapeutic molecules we and Daiichi would like to advance to later stages of drug development. Under terms of the agreement, we retain the rights to co-develop and co-promote products resulting from this collaboration in North America while Daiichi retains co-development and promotion rights in the remainder of the world.
Technology transfer agreement
In September 2000, we entered into a technology transfer agreement with Questcor Pharmaceuticals, Inc. and acquired the license and technology to a hepatitis C research program. Under the terms of this agreement, we paid a nonrefundable and noncreditable fee of $500,000, and are required to pay future milestones and royalties, and issued to Questcor 83,333 shares of series E preferred stock, which converted to 83,333 of common stock upon the completion of our initial public offering. We were also committed to invest a total of $2 million in research and development expenses over a two-year period through 2002. This committed spending level was achieved midway through 2002. The agreement terminates upon the expiration of the last patent within the agreement. We have accounted for the series E preferred stock at $9.00 per share based on the deemed fair value of its common stock at the date of grant. We have expensed the aggregate value of approximately
50
$1.2 million in September 2000 as the acquired technology is not yet fully developed and has no alternative use.
3. SIGNIFICANT CONCENTRATIONS
For the year ended December 31, 2002, Novartis, Johnson and Johnson, Daiichi and Pfizer accounted for 70%, 18%, 6% and 6% of total revenues, respectively. For the year ended December 31, 2001, Pfizer, Johnson and Johnson and Novartis accounted for 17%, 27% and 56% of total revenues, respectively. For the year ended December 31, 2000, Pfizer, Johnson & Johnson and Novartis accounted for 22%, 25% and 52% of total revenues, respectively. Accounts receivable relate mainly to these collaborative partners. The Company does not require collateral or other security for accounts receivable.
4. AVAILABLE-FOR-SALE SECURITIES
Available-for-sale securities consist of the following (in thousands):
| |
Amortized Cost and Fair Value at December 31, |
|||||
|---|---|---|---|---|---|---|
| |
2002 |
2001 |
||||
| Money market funds | $ | 26,535 | $ | 11,488 | ||
| Corporate commercial paper | 756 | 21,927 | ||||
| $ | 27,291 | $ | 33,415 | |||
| Reported as: | ||||||
| Cash and cash equivalents | $ | 26,535 | $ | 11,488 | ||
| Available-for-sale securities | 756 | 21,927 | ||||
| $ | 27,291 | $ | 33,415 | |||
At December 31, 2002, the available-for-sale securities had maturities of less than one year, with an average maturity of approximately 105 days.
There were no material gross realized gains or losses from sales of securities in the periods presented. Recorded unrealized gains and losses on available-for-sale securities were not material at December 31, 2002 or 2001.
5. PROPERTY AND EQUIPMENT
Property and equipment consists of the following (in thousands):
| |
Years Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2002 |
2001 |
|||||
| Laboratory and office equipment | $ | 16,691 | $ | 14,667 | |||
| Leasehold improvements | 3,175 | 3,169 | |||||
| Construction in progress | 197 | 592 | |||||
| Total property and equipment | 20,063 | 18,428 | |||||
| Less accumulated depreciation and amortization | (14,857 | ) | (9,988 | ) | |||
| Property and equipment, net | $ | 5,206 | $ | 8,440 | |||
51
At December 31, 2002 and 2001, equipment under capital leases was approximately $15.0 million and $15.2 million, respectively with accumulated depreciation and amortization of approximately $13.3 million and $8.5 million, respectively. Amortization expense was $1.7 million, $1.0 million, and $0.3 million for the years ended December 31, 2002, 2001, and 2000, respectively.
6. LONG-TERM OBLIGATIONS
At December 31, 2002, future minimum lease payments and obligations under all noncancelable leases were as follows (in thousands):
| |
Capital Leases |
Operating Leases |
||||
|---|---|---|---|---|---|---|
| 2003 | $ | 3,815 | $ | 7,169 | ||
| 2004 | 1,955 | 7,566 | ||||
| 2005 | 552 | 13,872 | ||||
| 2006 | — | 13,034 | ||||
| 2007 | — | 13,512 | ||||
| 2008 and thereafter | — | 142,975 | ||||
| Total minimum payments required | 6,322 | $ | 198,128 | |||
| Less amount representing interest | 621 | |||||
| Present value of future lease payments | 5,701 | |||||
| Less current portion | (3,388 | ) | ||||
| Noncurrent obligations under capital leases | 2,313 | |||||
During 2002, our office and research facility located at 240 East Grand in South San Francisco was leased under an operating lease terminated in conjunction with a 15-year lease for our current office and research facilities at 1180 Veterans Blvd. in South San Francisco signed in May 2001. Under the terms of the lease signed in 2001, we were to occupy our new facility in late 2002 and were to concurrently terminate our lease of our former facility at 240 East Grand in South San Francisco. We determined that the 2001 lease was an operating lease in accordance with FAS 13. In connection with the termination of the current 240 East Grand lease, we accelerated the amortization of tenant improvements and accrued rent charges over the expected remaining life of the lease and incurred minimal costs in connection with the terminated lease. The 1180 Veterans Blvd. research and office facilities were constructed as a build-to-suit facility. Under the original lease we were obligated to fund approximately $18.0 million of the total tenant improvement obligations. In October 2002, we amended this original lease to provide for a delay of the rent commencement date until February 1, 2003 and an increase in the tenant improvement allowance to cover the remaining construction obligations on the facility. The lease was also amended to increase the future rental commitments to compensate for the delay of the rent commencement and the increase in the tenant improvement allowance. Since the amendment was considered a material change to the original lease, we revisited the proper accounting treatment for this lease per FAS 13 and again determined the lease to be an operating lease. We moved into the new facilities during February 2003.
Prior to the signing of the amendment, we had been directly paying a portion of the pre-construction and construction costs related to the new facility. These costs were being capitalized on our balance sheet as construction-in progress. We have estimated that the landlord will be responsible for all of the costs that we had previously capitalized. Therefore, we have reclassified these costs into a short-term asset "Receivable from Landlord" and shown on the face of our balance sheet.
52
Rent expense under all operating leases amounted to approximately $1,897,000, $2,167,000 and $2,252,000 for the years ended December 31, 2002, 2001 and 2000, respectively.
In June 1998, we entered into a equipment lease line agreement for up to $3,000,000, which was fully utilized in June 1999. The lease period was for four years. The interest on each lease is fixed at the time of the draw down with the interest rates ranging from 6.5% to 7.2%.
In June 1999 and August 1999, we entered into two additional equipment lease line agreements for an aggregate total of $6,000,000, or $3,000,000 each additional lease agreement. These lines were fully utilized in May 2000. The lease period was for four years. The interest on each lease is fixed at the time of the draw down with the interest rates ranging from 11.7% to 15.0%.
In August 2000, we entered into an additional equipment lease line agreement for an aggregate total of $5,000,000. We utilized $4,148,000 of the facility but have no remaining availability under the facility. The lease period was for four years. The interest on the lease is fixed at the time of the draw down with the interest rates ranging from 10.6% to 14.6%.
In January 2002, we entered into an additional equipment lease line agreement for an aggregate total of $2,000,000. This line was fully utilized in August 2002. The lease period was for 37 months. The interest on the lease is fixed at the time of the draw down with the interest rates ranging from 11.5% to 11.7%.
In July 2002, we entered into an tenant improvement and equipment lease line agreement for an aggregate total of $15,000,000. Due to the amendment of our master lease agreement for our 1180 Veterans Blvd. facility signed in October 2002, we terminated the line without drawing down any of the available funds. Therefore, we do not have access to any amount under the line.
In December 2002, we entered into an additional equipment lease line agreement for an aggregate total of $2,000,000. We have the ability to draw down on this line until December 2003. As of December 31, 2002, no amounts under this line had been utilized. The lease period will be for three years. The interest on the lease is fixed at the time of any draw down.
Obligations under all leases are secured by the assets financed under the leases.
7. STOCKHOLDERS' EQUITY
Preferred and common stock
In February 2000, we completed a private placement of 2,508,330 shares of series E preferred stock at $6.00 per share for net proceeds of approximately $15.1 million. At the date of issuance, we believed the per share price of $6.00 represented the fair value of the preferred stock. Subsequent to the commencement of our initial public offering process, we re-evaluated the fair value of its common stock as of February 2000 and determined it to be $9.00 per share. Accordingly, the increase in fair value has resulted in a beneficial conversion feature of $10.0 million that has been recorded as a deemed dividend to the preferred stockholders in 2000. We recorded the deemed dividend at the date of issuance by offsetting charges and credits to additional paid-in-capital without any effect on total stockholders' equity. The preferred stock dividend increases the net loss allocable to common stockholders in the calculation of basic and diluted net loss per common share in 2000. Also in February 2000, we issued 50,000 shares of series E preferred stock for a license of technology. We valued the license at $500,000 and have expensed this amount in 2000 as the useful life is deemed to be less than one year.
In August 2000, we issued 33,333 shares of series E preferred stock to one of our directors. We recorded a deemed dividend of approximately $100,000 at the time of issuance.
53
In January 2002, we issued 7,000,000 shares of common stock in a registered direct offering to certain institutional investors at a price of $4.50 per share under our shelf registration statement. We received net proceeds of approximately $29.4 million after deducting commissions and offering costs. In February 2002, we issued 465,117 shares of common stock in a registered direct offering to a certain institutional investor at a price of $4.30 per share under our shelf registration statement. We received net proceeds of approximately $1.8 million after deducting commissions and offering costs.
Warrants
In conjunction with the equipment lease line executed in April 1997, we issued a warrant to purchase 175,000 shares of series B preferred stock at an exercise price of $0.80 per share. Upon the closing of our initial public offering, this warrant automatically converted to a warrant to purchase 175,000 shares of common stock at $0.80 per share. this warrant was exercised in June 2001 and was no longer outstanding as of December 31, 2001.
In conjunction with the equipment lease line executed in June 1998, we issued a warrant to purchase 131,578 shares of series C preferred stock at an exercise price of $1.14 per share. Upon the closing of our initial public offering, this warrant automatically converted to a warrant to purchase 131,578 shares of common stock at $1.14 per share. this warrants was exercised in June 2001 and is no longer outstanding as of December 31, 2001.
In conjunction with the facilities lease entered into in June 1998, we issued three warrants to purchase an aggregate of 150,000 shares of common stock at an exercise price of $1.14 per share. The warrants are exercisable at any time up to November 28, 2007, the seventh anniversary of the closing of our initial public offering.
In conjunction with the facilities lease entered into in May 2001, we issued a warrant to purchase 150,000 shares of our common stock at an exercise price of $8.91 per share, a 15% premium to market at the time of issuance. This warrant will expire on May 16, 2006. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $683,000. This amount has been capitalized in other long term assets and is being amortized into expense over the life of the lease.
In conjunction with the equipment lease line executed in January 2002, we issued a warrant to purchase 23,810 shares of our common stock at an exercise price of $4.20 per share. This warrant will expire on January 31, 2007. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $66,000. This amount has been capitalized in other long-term assets and is being amortized into expense over the payment period of the equipment lease line.
In conjunction with the equipment lease line executed in July 2002, we issued a warrant to purchase 138,889 shares of our common stock at an exercise price of $2.70 per share. This warrant will expire on July 12, 2012. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $251,000. This amount was completely expensed in 2002 in conjunction with the termination of the line.
In conjunction with the amendment of our master lease agreement for our 1180 Veterans Blvd. facility entered into in October 2002, we issued a warrant to purchase 500,000 shares of our common stock at an exercise price of $1.97 per share. This warrant will expire on October 18, 2007. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $565,000. This amount has been capitalized in other long term assets and is being amortized into expense over the life of the lease.
54
In conjunction with the equipment lease line executed in December 2002, we issued a warrant to purchase 186,916 shares of our common stock at an exercise price of $1.07 per share. This warrant will expire on December 23, 2007. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $136,000. This amount has been capitalized in other long-term assets and is being amortized into expense over the payment period of the equipment lease line.
Stock option plans
In January 2000, we adopted the 2000 Equity Incentive Plan (the "2000 Plan"), which was approved in March 2000 by our stockholders. The 2000 Plan is an amendment and restatement of the 1997 Stock Option Plan. Under the 2000 Plan, incentive stock options, nonstatutory stock options and shares of common stock may be granted to our employees, directors and consultants. As of December 31, 2002, a total of 6,320,000 shares of common stock have been authorized for issuance under the 2000 Plan.
In July 2001, we adopted the 2001 Non-Officer Equity Incentive Plan (the "2001 Plan"). Under the 2001 Plan, which was not approved by our stockholders, nonstatutory stock options may be granted to our employees and consultants. As of December 31, 2002, a total of 3,500,000 shares of common stock have been authorized for issuance under the 2001 Plan.
Options granted under our 2000 Plan and 2001 Plan expire no later than ten years from the date of grant. The option price of each incentive stock option shall be at least 100% of the fair value on the date of grant, and the option price for each nonstatutory stock option shall be not less than 85% of the fair value on the date of grant, as determined by the board of directors. Options may be granted with different vesting terms from time to time, not to exceed five years from the date of grant.
In August 2000, we adopted the 2000 Non-Employee Directors Stock Option Plan (the "Directors' Plan"), which was approved in September 2000 by our stockholders. Each non-employee director who becomes a director of Rigel will be automatically granted a nonstatutory stock option to purchase 20,000 shares of common stock on the date on which such person first becomes a director. At each board meeting immediately following each annual meeting of stockholders, beginning with the board meeting following the 2001 Annual Stockholders Meeting, each non-employee director will automatically be granted a nonstatutory option to purchase 5,000 shares of common stock. The exercise price of options under the Directors' Plan will be equal to the fair market value of the common stock on the date of grant. The maximum term of the options granted under the Directors' Plan is ten years. All grants under the Directors' Plan will vest monthly over two years from date of grant. The Directors' Plan will terminate in September 2009, unless terminated earlier in accordance with the provisions of the Directors' Plan. As of December 31, 2002, a total of 300,000 shares of common stock have been authorized for issuance under the Directors' Plan.
55
Activity under all of the option plans through December 31, 2002 was as follows:
| |
Shares Available For Grant |
Number of Options |
Weighted-Average Exercise Price |
|||||
|---|---|---|---|---|---|---|---|---|
| Outstanding at December 31, 1999 | 3,694,662 | 5,242,004 | $ | 0.19 | ||||
| Authorized for grant | 300,000 | — | ||||||
| Shares granted out of the plans | (100,000 | ) | 100,000 | — | ||||
| Granted | (2,563,609 | ) | 2,563,609 | 6.09 | ||||
| Exercised | — | (1,733,824 | ) | 0.16 | ||||
| Cancelled | 501,991 | (501,991 | ) | 3.47 | ||||
| Outstanding at December 31, 2000 | 1,833,044 | 5,669,798 | 2.70 | |||||
| Authorized for grant | 3,500,000 | — | — | |||||
| Granted | (1,031,901 | ) | 1,031,901 | 6.21 | ||||
| Exercised | — | (552,388 | ) | 0.57 | ||||
| Cancelled | 388,238 | (388,238 | ) | 3.05 | ||||
| Options outstanding at December 31, 2001 | 4,689,381 | 5,761,073 | 3.48 | |||||
| Granted | (1,662,916 | ) | 1,662,916 | 3.35 | ||||
| Exercised | — | (330,848 | ) | 0.26 | ||||
| Cancelled | 628,051 | (628,051 | ) | 4.93 | ||||
| Options outstanding at December 31, 2002 | 3,654,516 | 6,465,090 | $ | 3.47 | ||||
Details of the Company's stock options by exercise price is as follows:
| |
Options Outstanding |
|
Options Exercisable |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise Price |
Number of Outstanding Options |
Weighted-Average Remaining Contractual Life |
Weighted-Average Exercise Price |
Number of Options |
Weighted-Average Exercise Price |
|||||||
| $0.10 - $0.30 | 2,260,880 | 6.03 | $ | 0.20 | 1,598,180 | $ | 0.19 | |||||
| $1.40 - $3.00 | 646,331 | 9.78 | $ | 1.86 | 33,442 | $ | 1.91 | |||||
| $3.74 - $5.77 | 2,468,679 | 8.21 | $ | 4.62 | 1,144,464 | $ | 4.72 | |||||
| $7.50 - $11.00 | 1,089,200 | 7.77 | $ | 8.61 | 646,545 | $ | 8.64 | |||||
| $0.10 - $11.00 | 6,465,090 | 7.53 | $ | 3.47 | 3,422,631 | $ | 3.32 | |||||
The weighted-average fair value of the options granted in 2002, 2001 and 2000 was $2.27, $3.57 and $3.32, respectively.
We granted 65,000, 115,000 and 358,563 common stock options to consultants in exchange for services in 2002, 2001 and 2000, respectively. We have recorded compensation recovery related to these options of $196,000 and $510,000 for the years ended December 31, 2002 and 2001, respectively. We have recorded compensation expense related to these options of $5,280,000 for the year ended December 31, 2000. In accordance with SFAS 123 and EITF 96-18, options granted to consultants are periodically revalued as they vest. In January 2000, the Company recorded an expense of $664,000 related to the accelerated vesting of an option to purchase 75,000 shares of common stock issued to a consultant for services.
We have recorded deferred stock compensation with respect to options granted to employees of approximately $0.3 million and $4.9 million in the years ended December 31, 2001 and 2000, respectively, representing the difference between the exercise price of the options and the deemed fair value of the common stock on the date of the grant. These amounts are being amortized to operations over the vesting periods of the options using the graded vesting method. Such amortization expense amounted to approximately $1.7 million, $3.6 million and $4.9 million for the years ended
56
December 31, 2002, 2001 and 2000, respectively, and is expected to be approximately $0.7 million in 2003 and $0.1 million in 2004.
2000 employee stock purchase plan
In August 2000, we adopted the 2000 Employee Stock Purchase Plan (the "Purchase Plan"), which was approved in September 2000 by our stockholders. The Purchase Plan permits eligible employees to purchase common stock at a discount through payroll deductions during defined offering periods. The price at which the stock is purchased is equal to the lower of 85% of the fair market value of the common stock on the first day of the offering or 85% of the fair market value of our common stock on the purchase date. The initial offering period commenced on the effective date of our initial public offering. We issued 174,053 shares of common stock during 2002 and 120,458 shares of common stock during 2001 pursuant to the Purchase Plan at an average price of $2.07 per share in 2002 and $4.98 per share in 2001. For 2002 and 2001, the weighted average fair value of stock issued under the Purchase Plan was $1.68 and $2.42, respectively. A total of 400,000 shares of the Company's common stock were initially reserved for issuance under the Purchase Plan. The Purchase Plan provides for annual increases in the number of shares available for issuance under the Purchase Plan on each anniversary date of the effective date of the offering. The number of shares reserved automatically is equal to the lesser of 400,000 shares, 1% of the outstanding shares on the date of the annual increase or such amount as may be determined by the board. The number of shares reserved for future issuance under the Purchase Plan was increased by 400,000 during 2002 and 376,587 during 2001.
Reserved shares
As of December 31, 2002, we had reserved shares of common stock for future issuance as follows:
| |
December 31, 2002 |
||
|---|---|---|---|
| Warrants | 1,149,615 | ||
| Incentive stock plans. | 10,119,606 | ||
| Purchase Plan | 882,076 | ||
| Total | 12,151,297 | ||
8. INCOME TAXES
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred tax assets are as follows:
| |
Years Ended December 31, |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
2002 |
2001 |
||||||
| Deferred tax assets | ||||||||
| Net operating loss carryforwards | $ | 31,300 | $ | 18,500 | ||||
| Research and development credits | 5,500 | 3,100 | ||||||
| Capitalized research and development expenses | 3,500 | 2,000 | ||||||
| Other, net | 4,100 | 2,600 | ||||||
| Total deferred tax assets | 44,400 | 26,200 | ||||||
| Valuation allowance | (44,400 | ) | (26,200 | ) | ||||
| Net deferred tax assets | $ | — | $ | — | ||||
57
Realization of deferred tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance. The valuation allowance increased by $18.2 million, $5.2 million, and $10.2 million during 2002, 2001, and 2000, respectively.
Included in the valuation allowance balance is $1.6 million related to the exercise of stock options which are not reflected as an expense for financial reporting purposes. Accordingly, any future reduction in the valuation allowance relating to this amount will be credited directly to equity and not reflected as an income tax benefit in the statement of operations.
As of December 31, 2002, we had net operating loss carryforwards for federal income tax purposes of approximately $90.0 million, which expire in the years 2011 through 2022, and federal research and development tax credits of approximately $3.4 million, which expire in the years 2012 through 2022.
Utilization of the net operating loss and credit may be subject to a substantial annual limitation due to the "change in ownership" provisions of the Internal Revenue Code of 1986 (IRC) and similar state provisions. The annual limitation may result in the expiration of net operating losses and credits before utilization.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets.
9. SUBSEQUENT EVENTS
Reduction in Force
On January 31, 2003, we implemented a restructuring plan to reduce the rate of our cash consumption and better align our operating structure with current and expected future economic conditions. The restructuring plan included an immediate reduction in force of approximately 16 percent, or 25 employees, to 135 employees with reductions occurring in all functional areas. Two of our officers were included in this reduction in force.
10. SELECTED QUARTERLY FINANCIAL DATA (unaudited, in thousands, except per share amounts)
| |
Year Ended December 31, 2002 |
Year Ended December 31, 2001 |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Q1 |
Q2 |
Q3 |
Q4 |
Q1 |
Q2 |
Q3 |
Q4 |
|||||||||||||||||
| Revenue | $ | 4,098 | $ | 4,337 | $ | 3,653 | $ | 3,700 | $ | 3,194 | $ | 3,123 | $ | 4,206 | $ | 4,780 | |||||||||
| Net loss. | $ | (8,372 | ) | $ | (10,446 | ) | $ | (10,142 | ) | $ | (8,070 | ) | $ | (4,160 | ) | $ | (7,315 | ) | $ | (6,219 | ) | $ | (6,111 | ) | |
| Net loss per share to common stockholders, basic and diluted | $ | (0.19 | ) | $ | (0.23 | ) | $ | (0.22 | ) | $ | (0.18 | ) | $ | (0.11 | ) | $ | (0.20 | ) | $ | (0.17 | ) | $ | (0.16 | ) | |
| Weighted average shares used in computing net loss per common share, basic and diluted | 43,312 | 45,339 | 45,515 | 45,601 | 36,901 | 37,094 | 37,516 | 37,628 | |||||||||||||||||
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
58
Item 10. Directors and Executive Officers of the Registrant
Executive Officers and Directors
Set forth below is the name, age, position and a brief account of the business experience of each of our executive officers and directors as of February 15, 2003.
| Name |
Age |
Position |
||
|---|---|---|---|---|
| James M. Gower | 54 | Chief Executive Officer, Chairman of the Board and Director | ||
| Brian C. Cunningham(1) | 59 | President and Chief Operating Officer | ||
| Donald G. Payan, MD | 54 | Executive Vice President, Chief Scientific Officer and Director | ||
| James H. Welch | 45 | Vice President, Chief Financial Officer and Secretary | ||
| Raul R. Rodriguez | 42 | Senior Vice President, Business Development and Commercial Operations | ||
| Susan Molineaux, PhD(2) | 49 | Vice President, Biology | ||
| Elliott B. Grossbard, MD | 55 | Vice President of Medical Development | ||
| Dolly Vance | 38 | General Counsel and Vice President of Intellectual Property | ||
| Jean Deleage, PhD(3) | 62 | Director | ||
| Alan D. Frazier(4) | 50 | Director | ||
| Walter H. Moos, PhD(5) | 48 | Director | ||
| Stephen A. Sherwin, MD(6) | 54 | Director | ||
| Thomas S. Volpe(6) | 51 | Director |
James M. Gower has been our Chairman of the Board and Chief Executive Officer since October 2001. Mr. Gower joined us as our President, Chief Executive Officer and as a member of our board of directors in January 1997. From 1992 to March 1996, Mr. Gower was President and Chief Executive Officer of Tularik Inc., a biotechnology company developing small-molecule drugs regulating gene expression. Prior to Tularik, Mr. Gower spent ten years at Genentech, Inc., a biopharmaceutical company, where he most recently served as Senior Vice President. During his ten years at Genentech, Mr. Gower was responsible for business development and sales and marketing functions. In addition, he established and managed Genentech's foreign operations in Canada and Japan and served as President of Genentech Development Corporation. Mr. Gower serves on the board of directors of Cell Genesys, Inc. He holds a BS and an MBA in operations research from the University of Tennessee.
Brian C. Cunningham left Rigel in January 2003 and had been our President and Chief Operating Officer since October 2001. Mr. Cunningham was our Secretary from July 1996 to October 2001. In
59
July 1998, he joined us as Senior Vice President and Chief Operating Officer, and from February 1999 until October 2001, he was our Chief Financial Officer. From January 1989 to September 1998, Mr. Cunningham was a partner in the law firm Cooley Godward LLP, where he was head of the Life Sciences Group and the Health Care Group. From May 1982 to December 1989, he served as Vice President, Secretary and General Counsel of Genentech Inc. Mr. Cunningham holds a BS and a JD from Washington University.
Donald G. Payan, MD is our co-founder, has been a member of our board of directors since July 1996 and has served as our Executive Vice President and Chief Scientific Officer since January 1997. From January 1997 to July 1998, he also served as our Chief Operating Officer. From July 1996 to January 1997, Dr. Payan served as our President and Chief Executive Officer. From December 1995 to May 1996, Dr. Payan was Vice President of AxyS Pharmaceuticals, Inc., a biopharmaceutical company. From September 1993 to December 1995, Dr. Payan was the founder and Executive Vice President and Chief Scientific Officer of Khepri Pharmaceuticals, Inc., which merged with AxyS Pharmaceuticals. Dr. Payan continues his association with the University of California, San Francisco, which began in 1982, where he is currently an Adjunct Professor of Medicine and Surgery. Dr. Payan holds a BS and an MD from Stanford University.
James H. Welch has been our Vice President, Chief Financial Officer and Secretary since October 2001. Mr. Welch joined us as our Vice President, Finance and Administration and Assistant Secretary in May 1999. From June 1998 to May 1999, he served as an independent consultant at various companies. From February 1997 to June 1998, Mr. Welch served as Chief Financial Officer of Biocircuits Corporation, a manufacturer of medical diagnostic equipment, and from June 1992 to February 1997, he served as Corporate Controller of Biocircuits. Previously, Mr. Welch held various positions at NeXT Computer, Inc., most recently as Division Controller. Mr. Welch holds a BA from Whitworth College and an MBA from Washington State University.
Raul R. Rodriguez joined us as our Vice President, Business Development in April 2000 and became our Senior Vice President, Business Development and Commercial Operations in December 2002. From 1997 to March 2000, he served as Senior Vice President, Business Development and Operations for Ontogeny, Inc., a biotechnology company. From 1994 to 1997, he served as the Executive Director, Business Development and Market Planning for Scios, Inc., a pharmaceutical company. From 1989 to 1994 Mr. Rodriguez held various positions at Searle Pharmaceuticals. Mr. Rodriguez holds an AB from Harvard University, an MPH from the University of Illinois and an MBA from Stanford University.
Susan Molineaux, PhD left Rigel in January 2003 and had been our Vice President, Biology since January 2002. Dr. Molineaux joined us as our Senior Director, Combinatorial Biology and Drug Discovery in February 2000. From 1999 to 2000, Dr. Molineaux served as Vice President of Biology at Praelux Incorporated, a biotechnology company. From 1994 to 1999, she served as Vice President of Drug Development Research at Praecis Pharmaceuticals. From 1989 to 1992, she served as Senior Research Immunologist in the Immunology Department at Merck and Co. Dr. Molineaux holds a BA from Smith College and a PhD in genetics from Johns Hopkins University.
Elliott B. Grossbard, MD joined us as Senior Vice President of Medical Development in April 2002. Prior to joining Rigel, Dr. Grossbard was Vice President, Clinical Affairs for Avigen Inc., a gene therapy products company. Before that, Dr. Grossbard served as Senior Vice President of Development and Vice President of Medical and Regulatory Affairs at Scios, Inc. From 1982 through 1990, Dr. Grossbard held the positions of Associate Director of Clinical Research and Director of Clinical Research at Genentech Inc. Dr. Grossbard holds a BA from Columbia College, an MD from Columbia University and an MS in Law from Yale University School of Law.
Dolly Vance has been our General Counsel and Vice President of Intellectual Property since January 2003. Ms. Vance joined us as Senior Patent Attorney in September 2000, and from
60
January 2002 until December 2002, she served as Associate General Counsel and Director of Intellectual Property. From 1997 until 2000, she was an attorney with the law firm of Flehr Hohbach Test Albritton & Herbert, where she last held the position of partner. From 1995 until 1997, Ms. Vance was an associate at the law of firm of Arnall Golden & Gregory, and from 1993 to 1995, she was an associate with the law firm of Harness Dickey & Pierce. Ms. Vance holds a BA from the Unviersity of California, San Diego and a JD from Boston University School of Law.
Jean Deleage, PhD joined us as a director in January 1997. Dr. Deleage is a founder and managing director of Alta Partners, a venture capital firm investing in information technologies and life science companies. Dr. Deleage is a managing partner of Burr, Egan, Deleage & Co., a venture capital firm that he founded in 1979. Dr. Deleage was a founder of Sofinnova, a venture capital organization in France, and Sofinnova, Inc., the U.S. subsidiary of Sofinnova. Dr. Deleage currently serves on the board of directors of Crucell, N.V. and Kosan Biosciences Incorporated. Dr. Deleage received a Baccalaureate in France, a Masters Degree in electrical engineering from the Ecole Superieure d'Electricite and a PhD in economics from the Sorbonne.
Alan D. Frazier joined us as a director in October 1997. In 1991, Mr. Frazier founded Frazier Healthcare Ventures, a venture capital firm, and has served as the managing principal since its inception. From 1983 to 1991, Mr. Frazier served as Executive Vice President, Chief Financial Officer and Treasurer of Immunex Corporation, a biopharmaceutical company. From 1980 to 1983, Mr. Frazier was a principal in the Audit Department of Arthur Young & Company (now Ernst & Young). He also serves on the board of trustees of the Fred Hutchinson Cancer Research Center. Mr. Frazier holds a BA in economics from the University of Washington.
Walter H. Moos, PhD joined us as a director in March 1997. Since 1997, Dr. Moos has served as the Chairman and Chief Executive Officer of MitoKor, a biotechnology company. From 1991 to 1997, he served as Corporate Vice President and Vice President, Research and Development in the Technologies Division of Chiron Corporation, a biotechnology company. From 1982 to 1991, Dr. Moos held several positions at the Parke-Davis Pharmaceutical Research Division of the Warner-Lambert Company, last holding the position of Vice President, Neuroscience and Biological Chemistry. He has been an Adjunct Professor at the University of California, San Francisco, since 1992. Dr. Moos holds an AB from Harvard University and a PhD in chemistry from the University of California, Berkeley.
Stephen A. Sherwin, MD joined us as a director in March 2000. Since March 1990, he has served as Chief Executive Officer and director of Cell Genesys, Inc., and as Chairman of the Board of Cell Genesys since March 1994. From March 1990 to August 2001, Dr. Sherwin held the additional position of President of Cell Genesys. From 1983 to 1990, Dr. Sherwin held various positions at Genentech Inc., a biopharmaceutical company, most recently as Vice President, Clinical Research. Dr. Sherwin currently serves as Chairman of the Board of Ceregene, Inc., a majority-owned subsidiary of Cell Genesys, and as a director of Neurocrine Biosciences, Inc. He received his MD from Harvard Medical School and his BA from Yale University.
Thomas S. Volpe joined us as a director in August 2000. Mr. Volpe is the Chairman and Chief Executive Officer of Volpe Investments, LLC, a risk capital investment firm. Until May 2001, he was the Chairman of Prudential Volpe Technology Group. From 1986 to 1999, Mr. Volpe was President, Chief Executive Officer and founder of Volpe Brown Whelan & Company, a risk capital and investment banking firm. Prior to forming Volpe Brown Whelan & Company, he was President, Chief Executive Officer and a member of the board of directors and management committee of Hambrecht & Quist Incorporated. Before joining Hambrecht & Quist, Mr. Volpe was Head of the Science and Technology Group of Blyth Eastman PaineWebber. Mr. Volpe also serves on the board of directors of Linear Technology Corporation. Mr. Volpe holds an AB in economics from Harvard University, an MSc in economics from the London School of Economics and an MBA from the Harvard Business School.
61
Our executive officers are appointed by our board of directors and serve until their successors are elected or appointed. There are no family relationships among any of our directors or executive officers. No director has a contractual right to serve as a member of our board of directors.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934 requires our directors and executive officers, and persons who own more than ten percent of a registered class of our equity securities, to file with the SEC initial reports of ownership and reports of changes in ownership of our common stock and other equity securities. Officers, directors and greater than ten percent stockholders are required by SEC regulation to furnish us with copies of all Section 16(a) forms that they file.
To our knowledge, based solely on a review of the copies of such reports furnished to us and written representations that no other reports were required, during the fiscal year ended December 31, 2002, all Section 16(a) filing requirements applicable to our officers, directors and greater than ten percent beneficial owners were complied with.
Item 11. Executive Compensation
The following table sets forth information concerning the compensation that we paid during the fiscal years ended December 31, 2002, 2001 and 2000 to our Chief Executive Officer and each of the four other most highly compensated executive officers who earned more than $100,000 during 2002.
| |
|
|
|
Long Term Compensation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Annual Compensation |
|||||||||||||
| Name and Principal Position |
Securities Underlying Options/SARS(1) |
All other Compensation |
||||||||||||
| Year |
Salary |
Bonus |
||||||||||||
| James M. Gower Chief Executive Officer, Chairman of the Board and Director |
2002 2001 2000 |
$ |
330,000 288,837 267,800 |
$ |
— 50,000 — |
— — — |
— — — |
|||||||
Brian C. Cunningham(2) President and Chief Operating Officer |
2002 2001 2000 |
300,000 269,626 257,500 |
— 50,000 — |
— — 200,000 |
— — — |
|||||||||
Donald G. Payan Executive Vice President and Chief Scientific Officer and Director |
2002 2001 2000 |
300,000 263,833 247,200 |
— 60,000 — |
— — — |
— — — |
|||||||||
Raul Rodriguez(3) Senior Vice President, Business Development and Commercial Operations |
2002 2001 2000 |
240,000 216,321 165,000 |
— 15,000 — |
150,000 — 245,000 |
$ |
— — 12,226 |
(4) |
|||||||
Elliot B. Grossbard(5) Senior Vice President, Medical Development |
2002 2001 2000 |
206,270 — — |
— — — |
250,000 — — |
— — — |
|||||||||
62
Stock Option Grants and Exercises
The following table sets forth summary information regarding the option grants made to our Chief Executive Officer and each of our four other most highly paid executive officers during 2002. Options granted to purchase shares of our common stock under our 2000 Equity Incentive Plan generally vest over a four-year period. The exercise price per share is equal to the fair market value of our common stock on the date of grant.
The potential realizable value is calculated based on the ten-year term of the option at the time of grant. Stock price appreciation of 5% and 10% is assumed pursuant to rules promulgated by the SEC and does not represent our prediction of our stock price performance. The potential realizable values at 5% and 10% appreciation are calculated by:
Percentages shown under "% of Total Options Granted to Employees in 2002" are based on an aggregate of 1,662,916 options granted to employees under our 2000 Equity Incentive Plan and our 2001 Non-Officer Equity Incentive Plan during 2002.
Option Grants in Last Fiscal Year Ended December 31, 2002
| |
|
Individual Grants |
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of Securities Underlying Options Granted |
% of Total Options Granted to Employees in 2002 |
|
|
Potential Realizable Value at Assumed Annual Rates of Appreciation of Stock Price for Option Term |
||||||||
| Name |
Exercise Price $/Sh |
Expiration Date |
|||||||||||
| 5% |
10% |
||||||||||||
| James M. Gower | — | — | — | — | — | — | |||||||
| Donald G. Payan | — | — | — | — | — | — | |||||||
| Brian C. Cunningham | — | — | — | — | — | — | |||||||
| Raul Rodriguez | 150,000 | 9.0 | % | $ | 1.40 | 11/22/12 | — | — | |||||
| Elliott B. Grossbard | 250,000 | 15.0 | % | 3.74 | 4/9/12 | 54,477 | 213,963 | ||||||
The following table sets forth summary information regarding the number and value of shares acquired upon exercise of options in 2002 and options held as of December 31, 2002 for our Chief Executive Officer and each of our four most highly compensated executive officers. Amounts shown in the "Value of Unexercised In-the-Money Options at December 31, 2002" column are based on the closing market price on December 31, 2002 of $1.10 per share, without taking into account any taxes that may be payable in connection with the transaction, multiplied by the number of shares underlying the option, less the aggregate exercise price payable for the shares.
63
Aggregated Option Exercises in Last Fiscal Year and Fiscal Year-End Option Values
| |
|
|
Number of Securities Underlying Unexercised Options at December 31, 2002 |
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
Value of Unexercised In-the-Money Options at December 31, 2002 |
|||||||||||
| Name |
Shares Acquired on Exercise (#) |
Value Realized |
||||||||||||
| Vested |
Unvested |
Vested |
Unvested |
|||||||||||
| James M. Gower | — | — | 345,000 | 105,000 | $ | 310,500 | $ | 94,500 | ||||||
| Donald G. Payan. | — | — | 115,000 | 35,000 | 103,500 | 31,500 | ||||||||
| Brian C. Cunningham(1) | — | — | 587,449 | 112,501 | 430,341 | 438,002 | ||||||||
| Raul Rodriguez | — | — | 176,666 | 218,334 | — | — | ||||||||
| Elliott B. Grossbard | — | — | — | 250,000 | — | — | ||||||||
Compensation of Directors
Rigel does not provide cash compensation to members of its board of directors for serving on the board of directors or for attendance at committee meetings. The members of the board of directors are eligible for reimbursement for their expenses incurred in connection with attendance at board meetings in accordance with Rigel policy.
Each of our non-employee directors receives stock option grants under the 2000 Non-Employee Directors' Stock Option Plan, or Directors' Plan. Only non-employee directors or their affiliates are eligible to receive options under the Directors' Plan. Options granted under the Directors' Plan are not intended to qualify as incentive stock options under the Internal Revenue Code of 1986, as amended.
Option grants under the Directors' Plan are non-discretionary. Each person who is elected or appointed for the first time to be a non-employee director automatically receives, upon the date of his or her initial election or appointment to be a non-employee director by the board or Rigel stockholders, an initial grant to purchase 20,000 shares of common stock on the terms and conditions set forth in the plan. In addition, on the day following the annual meeting of stockholders each year, each non-employee director who continues to serve as a non-employee director automatically receives an annual option to purchase 5,000 shares of common stock. No other options may be granted at any time under the Directors' Plan. The exercise price of options granted under the Directors' Plan is 100% of the fair market value of our common stock on the date of the option grant. The options vest over two years in equal monthly installments provided that the non-employee director continues to provide services to Rigel. The term of options granted under the Directors' Plan is ten years. In the event of a merger of Rigel with or into another corporation or a consolidation, acquisition of assets or other change-in-control transaction involving us, each option either will continue in effect, if we are the surviving entity, or if neither assumed nor substituted, will accelerate and the option will terminate if not exercised prior to the consummation of the transaction.
Pursuant to the Directors' Plan, on June 21, 2002, the day after our 2002 annual meeting of stockholders, we granted options covering 5,000 shares of common stock to each of Drs. Deleage, Moos and Sherwin and Messrs. Volpe and Frazier, each at an exercise price of $3.00 per share. These options vest in 24 equal monthly installments beginning on the grant date.
Employment Contracts and Termination of Employment and Change of Control Arrangements
We have an employment agreement with Dr. Payan, our Executive Vice President and Chief Scientific Officer, dated as of January 16, 1997, which was amended in March 2003 and continues indefinitely. Pursuant to the terms of the amended agreement, Dr. Payan is entitled to receive an annualized base salary of $185,000 and was issued 750,000 shares of our common stock. As of
64
January 16, 2000, all such shares were fully vested and not subject to a right of repurchase by us. Either Rigel or Dr. Payan may terminate his employment at any time for any reason. If we terminate Dr. Payan's employment without cause, he will receive a severance payment equal to his annual base salary in effect at the date of termination.
We have an employment agreement with Dr. Grossbard, our Senior Vice President and Medical Director, dated as of March 18, 2002, and continuing indefinitely. Pursuant to the terms of the agreement, Dr. Grossbard is entitled to receive an annualized base salary of $275,000 and was issued an option to purchase 250,000 shares of our common stock. As of March 31, 2003, options to purchase 62,500 shares were fully vested and exercisable. Either Rigel or Dr. Grossbard may terminate his employment at any time for any reason. If, solely as a result of change in control of Rigel, Dr. Grossbard's employment is terminated or his responsibilities are substantially diminished for any reason prior to April 1, 2004, then the option to purchase 250,000 shares shall vest and become immediately exercisable in full.
Compensation Committee Interlocks and Insider Participation
Our Compensation Committee currently consists of two non-employee directors: Drs. Deleage and Moos. No member of the Compensation Committee is currently, or ever has been, an officer or employee of Rigel. No executive officer of Rigel has served as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our board of directors or Compensation Committee.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table provides certain information with respect to all of our equity compensation plans in effect as of December 31, 2002.
Equity Compensation Plan Information
| Plan Category |
Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) |
Weighted-average exercise price of outstanding options, warrants and rights (b) |
Number of securities remaining available for issuance under equity compensation plans (excluding securities reflected in column (a)) (c) |
||||
|---|---|---|---|---|---|---|---|
| Equity compensation plans approved by security holders | 5,451,302 | $ | 3.43 | 1,168,302 | |||
| Equity compensation plans not approved by security holders | 1,013,786 | $ | 3.70 | 2,486,214 | |||
| Total | 6,465,090 | $ | 3.47 | 3,654,516 | |||
In July 2001, we adopted our 2001 Non-Officer Equity Incentive Plan without the approval of our stockholders. Under this plan, nonstatutory stock options may be granted to our employees and consultants. As of December 31, 2002, a total of 3,500,000 shares of common stock had been authorized for issuance under the 2001 Non-Officer Equity Incentive Plan. Options granted under Non-Officer Equity Incentive Plan expire no later than ten years from the date of grant. The option price for each nonstatutory stock option shall be not less than 85% of the fair value on the date of grant. Options may be granted with different vesting terms from time to time but not to exceed five years from the date of grant.
65
The following table shows information known to us with respect to the beneficial ownership of our common stock as of February 15, 2003, by:
Beneficial ownership of shares is determined under the rules of the Securities and Exchange Commission and generally includes any shares over which a person exercises sole or shared voting or investment power. Except as indicated by footnote, and subject to applicable community property laws, each person identified in the table possesses sole voting and investment power with respect to all shares of common stock held by them. Shares of common stock subject to options currently exercisable or exercisable within 60 days of February 15, 2003 and not subject to repurchase as of that date are deemed outstanding for calculating the percentage of outstanding shares of the person holding these options, but are not deemed outstanding for calculating the percentage of any other person. Applicable percentage ownership in the following table is based on 45,851,496 shares of common stock outstanding
66
as of February 15, 2003. Unless otherwise indicated, the address of each of the named individuals is c/o Rigel Pharmaceuticals, Inc., 1180 Veterans Blvd., South San Francisco, California 94080.
| Beneficial Owner |
Outstanding Shares of Common Stock |
Shares Issuable Pursuant to Options Exercisable Within 60 Days of February 15, 2003 |
Percent of Total Outstanding Shares Beneficially Owned |
||||
|---|---|---|---|---|---|---|---|
| Five percent stockholders | |||||||
| Entities affiliated with Lombard Darier Hentsch & Cie(1) 11, rue de la Corraterie 1204 Geneva Switzerland |
6,269,538 | — | 13.7 | % | |||
| Entities affiliated with Alta Partners(2) One Embarcadero Center, Suite 4050 San Francisco, CA 94111 |
5,832,923 | — | 12.7 | ||||
| Entities affiliated with Frazier and Company, Inc.(3) 601 Union Street, Suite 2110 Seattle, WA 98101 |
4,347,719 | — | 9.5 | % | |||
| Novartis Pharma AG Head Financial Investments CH-4002 Basil, Switzerland |
3,428,571 | — | 7.5 | % | |||
Directors and named executive officers |
|||||||
| James M. Gower | 613,100 | 375,000 | 2.1 | % | |||
| Brian C. Cunningham(4) | 209,345 | 599,998 | 1.7 | % | |||
| Donald G. Payan, MD | 767,791 | 125,000 | 1.9 | % | |||
| Raul Rodriguez | 6,621 | 206,458 | * | ||||
| Elliott B. Grossbard, MD | — | 62,500 | * | ||||
| Jean Deleage, PhD(2) | 5,832,923 | 6,041 | 12.7 | % | |||
| Alan D. Frazier(3) | 4,347,719 | 1,875 | 9.5 | % | |||
| Walter H. Moos PhD | — | 26,041 | * | ||||
| Stephen A. Sherwin, MD | — | 32,382 | * | ||||
| Thomas S. Volpe | 33,333 | 26,041 | * | ||||
| All executive officers and directors as a group (13 people) | 11,862,944 | 1,888,959 | 30.0 | % |
67
Item 13. Certain Relationships and Related Transactions
Lombard Odier Darier Hentsch & Cie, Alta California Partners, L.P., Alta Embarcadero Partners, LLC, Frazier Healthcare II, L.P., Frazier and Company, Inc., Johnson and Johnson, Novartis and Thomas Volpe are entitled to certain rights with respect to registration under the Securities Act of shares of our common stock that they hold. These rights are provided under an Amended and Restated Investor Rights Agreement, dated February 3, 2000, and under agreements with similar registration rights. If we propose to register any of our securities under the Securities Act, either for our own account or for the account of others, these holders are entitled to notice of the registration and are entitled to include, at our expense, their shares of common stock in the registration and any related underwriting, provided, among other conditions, that the underwriters may limit the number of shares to be included in the registration. In addition, these holders may require us, at our expense and on not more than two occasions, to file a registration statement under the Securities Act with respect to their shares of common stock, and we will be required to use our best efforts to effect the registration. Further, these holders may require us at our expense to register their shares on Form S-3, subject to certain limitations. Pursuant to the registration rights set forth in Section 2.4 of the Amended and Restated Investor Rights Agreement., we registered an aggregate of 17,673,751 shares of common stock held by Lombard Odier Darier Hentsch & Cie, Alta California Partners, L.P., Alta Embarcadero Partners, LLC, Frazier Healthcare II, L.P., Frazier and Company, Inc. and Novartis. These shares were registered on a Registration Statement on Form S-3 filed with the SEC on April 30, 2002 (File No. 333-87276) and declared effective by the SEC on May 8, 2002.
We have entered into indemnification agreements with our directors and certain officers for the indemnification and advancement of expenses to these persons to the fullest extent permitted by law. We also intend to enter into those agreements with our future directors and officers.
In September 1999, we established a research collaboration and license agreement with Cell Genesys, Inc. that ended in 2002. James Gower, our President and Chief Executive Officer, serves on the board of directors of Cell Genesys. Stephen A. Sherwin, MD, who serves on our board of directors, is Chief Executive Officer and Chairman of the Board of Cell Genesys.
We have an employment agreement with Dr. Payan, our Executive Vice President and Chief Scientific Officer, dated as of January 16, 1997, and continuing indefinitely. Under the agreement, Dr. Payan is entitled to receive an annualized base salary of $185,000 and was issued 750,000 shares of our common stock. As of January 16, 2000, all such shares were fully vested and not subject to a right of repurchase by us. Either Rigel or Dr. Payan may terminate his employment at any time for any reason. If we terminate Dr. Payan's employment without cause, he will receive a severance payment equal to one year's base salary.
We have an employment agreement with Dr. Grossbard, our Senior Vice President and Medical Director, dated as of March 18, 2002, and continuing indefinitely. Pursuant to the terms of the agreement, Dr. Grossbard is entitled to receive an annualized base salary of $275,000 and was issued an option to purchase 250,000 shares of our common stock. As of March 31, 2003, options to purchase 62,500 shares were fully vested and exercisable. Either Rigel or Dr. Grossbard may terminate his employment at any time for any reason. If, solely as a result of change in control of Rigel, Dr. Grossbard's employment is terminated or his responsibilities are substantially diminished for any reason prior to April 1, 2004, then the option to purchase 250,000 shares shall vest and become immediately exercisable in full.
In May 1999, we signed an agreement for the establishment of a broad collaboration with Novartis, whereby the two companies agreed to work on up to five different five-year research projects to identify drug targets for products that can treat, prevent or diagnose the effects of human disease. According to the terms of the original agreement, two of the research projects were to be conducted jointly by Novartis and us, and the other three research projects were to be conducted at Novartis.
68
Four projects are now underway. The first research project, a joint research project, is focused on identifying small molecule drug targets that regulate T cells. The second research project, also a joint research project, relates to the identification and validation of small molecule drug targets that can mediate specific functions of B cells. The third research project, a project carried out at Novartis, is focused on identifying small molecule drug targets that regulate chronic bronchitis. In July 2001, Novartis and Rigel amended the agreement to add a three-year joint project at Rigel in the area of angiogenesis in lieu of a project at Novartis. In contrast to the original agreement to conduct an additional project at Novartis, this amendment resulted in both funded research at Rigel and an additional upfront payment to us of $4.0 million. In January 2002, Novartis chose not to exercise its option to add a second project to be conducted at Novartis. During 2002, Novartis notified us that it was terminating the research phase of the initial T Cell and B Cell joint projects after forty-two months. The termination dates for the research phases of the initial joint projects were therefore November 2002 and February 2003, respectively. The third research project, a project carried out at Novartis, is focused on identifying small molecule drug targets that regulate chronic bronchitis.
We believe that all of the transactions set forth above were made on terms no less favorable to us than could have been obtained from unaffiliated third parties. All future transactions, including loans, between us and our officers, directors, principal stockholders and their affiliates will be approved by a majority of our board of directors, including a majority of the independent and disinterested directors, and will be on terms no less favorable to us than could be obtained from unaffiliated third parties.
Item 14. Controls and Procedures
Evaluation of Disclosure Controls and Procedures. Our chief executive officer and chief financial officer have concluded that our disclosure controls and procedures, as defined Exchange Act Rule 13a-14(c), are sufficiently effective to ensure that the information required to be disclosed by us in the reports we file under the Exchange Act is gathered, analyzed and disclosed with adequate timeliness, accuracy and completeness, based on an evaluation of such controls and procedures conducted within 90 days prior to the date hereof.
Changes in Internal Controls. There have been no significant changes in our internal controls or in other factors that could significantly affect these controls subsequent to the date of the evaluation referred to above, nor were there any significant deficiencies or material weaknesses in our internal controls. Accordingly, no corrective actions were required or undertaken.
Limitations on the Effectiveness of Controls. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the controls are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within a company have been detected.
69
Item 15. Exhibits, Financial Statement Schedules and Reports on Form 8-K
(a) The following documents are being filed as part of this report:
| 3.1(1) | Amended and Restated Certificate of Incorporation. | |
| 3.2(1) | Amended and Restated Bylaws. | |
| 4.1(1) | Specimen Common Stock Certificate. | |
| 4.2(1) | Amended and Restated Investor Rights Agreement, dated February 3, 2000, between Rigel and holders of Rigel's Series B, Series C, Series D and Series E preferred stock. | |
| 4.3(1) | Form of warrant to purchase shares of common stock. | |
| 4.7(11) | Amended and Restated Warrant issued to Kwacker Limited for the purchase of shares of common stock. | |
| 4.8(7) | Warrant issued to TBCC Funding Trust II for the purchase of shares of Common Stock. | |
| 4.9(8) | Warrant issued to Comerica Bank-California for the purchase of shares of Common Stock | |
| 4.10(11) | Warrant issued to Kwacker Limited for the purchase of shares of common stock. | |
| 4.11(11) | Warrant issued to Lighthouse Capital Partners IV, L.P. to purchase shares of common stock. | |
| 10.1(1) | Form of Indemnity Agreement. | |
| 10.2(11)(2) | 2000 Equity Incentive Plan, as amended. | |
| 10.3(1)(2) | Form of Stock Option Agreement pursuant to 2000 Equity Incentive Plan. | |
| 10.4(1)(2) | 2000 Employee Stock Purchase Plan. | |
| 10.5(1)(2) | 2000 Non-Employee Directors' Stock Option Plan. | |
| 10.6(1) | Collaboration Agreement between Rigel and Janssen Pharmaceutica N.V., dated December 4, 1998. | |
| 10.7(1) | Collaborative Research and License Agreement between Rigel and Pfizer Inc., dated January 31, 1999. | |
| 10.8(1) | Collaboration Agreement between Rigel and Novartis Pharma AG, dated May 26, 1999. | |
| 10.9(1)(3) | License and Research Agreement between Rigel and Cell Genesys, Inc., dated September 2, 1999. | |
| 10.10(1) | Collaborative Research and Development Agreement between Rigel and Neurocrine Biosciences, Inc., dated December 1997. | |
| 10.11(1)(2) | Employment Agreement between Rigel and Donald Payan, dated January 16, 1997. | |
| 10.12(1) | Lease between Rigel and Britannia Pointe Grand Limited Partnership, dated June 2, 1998. | |
| 10.13(1) | Technology Transfer Agreement between Rigel and Questcor Pharmaceuticals, Inc., dated September 22, 2000. | |
| 10.14(3)(4) | License and Research Agreement (Amended and Restated) between Rigel and Cell Genesys, Inc., dated September 2, 1999, as amended and restated on March 26, 2001. | |
| 10.15(5) | Lease termination agreement between Rigel and Brittannia Pointe Grand Limited Partnership, dated May 6, 2001. | |
| 10.16(5) | Build-to-suit lease between Rigel and Slough BTC, LLC, dated May 16, 2001. | |
70
| 10.17(5) | First amendment to the Collaboration Agreement between Rigel and Novartis Pharma AG, dated May 18, 2001. | |
| 10.18(3)(6) | Second Amendment, dated July 6, 2001, to the Collaboration Agreement between Rigel and Novartis Pharma AG. | |
| 10.19(3)(6) | Second Amendment, dated July 1, 2001, to the Collaboration Agreement between Rigel and Cell Genesys, Inc. | |
| 10.20(2)(11) | 2001 Non-Officer Equity Incentive Plan, as amended. | |
| 10.21(2)(7) | Form of Stock Option Agreement pursuant to the 2001 Non-Officer Equity Incentive Plan. | |
| 10.22(8) | First Amendment, dated June 30, 2000, to the Collaboration Agreement by and between Rigel and Janssen Pharmaceutica N.V. | |
| 10.23(8) | Second Amendment, dated December 4, 2001, to the Collaboration Agreement by and between Rigel and Janssen Pharmaceutica N.V. | |
| 10.24(10) | Loan and Security Agreement between Rigel and Comerica Bank—California, dated July 12, 2002. | |
| 10.25(10)(3) | Collaboration Agreement between Rigel and Daiichi Pharmaceutical Co., Ltd., dated August 1, 2002. | |
| 10.26(11)(3) | Amendment to Build-to-suit lease between Rigel and Slough BTC, LLC, dated October 18, 2002. | |
| 10.27(11) | Master Lease Agreement between Rigel and Lighthouse Capital Partners IV, L.P., dated December 23, 2002. | |
| 10.28(2)(12) | Employment Agreement between Rigel and Elliott B. Grossbard, dated as of March 18, 2002. | |
| 10.29(2)(12) | Amendment to Employment Agreement, dated as of March 5, 2003, between Rigel and Donald Payan. | |
| 23.1(12) | Consent of Ernst & Young LLP, Independent Auditors. | |
| 24.1(11) | Power of Attorney. | |
| 99.1(12)(13) | Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
71
(b) We did not file any reports on Form 8-K during the fourth quarter of 2002.
(c) Exhibits
See Item 15(a) above
(d) Financial Data Schedules
See Item 15(a) above
72
Pursuant to the requirements of the Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report on Form 10-K/A to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of South San Francisco, State of California, on May 8, 2003.
| Rigel Pharmaceuticals, Inc. | |||
By: |
/s/ JAMES M. GOWER James M. Gower Chairman of the Board and Chief Executive Officer |
||
By: |
/s/ JAMES H. WELCH James H. Welch Vice President, Chief Financial Officer and Secretary |
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature |
Title |
Date |
||
|---|---|---|---|---|
| /s/ JAMES M. GOWER James M. Gower |
Chairman of the Board, Chief Executive Officer and Director (Principal Executive Officer) |
May 8, 2003 | ||
/s/ JAMES H. WELCH James H. Welch |
Vice President, Chief Financial Officer, and Secretary (Principal Finance and Accounting Officer) |
May 8, 2003 |
||
* Donald G. Payan |
Executive Vice President, Chief Scientific Officer and Director |
May 8, 2003 |
||
* Jean Deleage |
Director |
May 8, 2003 |
||
* Alan D. Frazier |
Director |
May 8, 2003 |
||
* Walter H. Moos |
Director |
May 8, 2003 |
||
* Stephen A. Sherwin |
Director |
May 8, 2003 |
||
73
* Thomas S. Volpe |
Director |
May 8, 2003 |
* |
/s/ JAMES H. WELCH James H. Welch Attorney-in-fact |
74
I, James M. Gower, certify that:
1. I have reviewed this annual report on Form 10-K/A of Rigel Pharmaceuticals, Inc.;
2. Based on my knowledge, this annual report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this annual report;
3. Based on my knowledge, the financial statements, and other financial information included in this annual report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this annual report;
4. The registrant's other certifying officers and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-14 and 15d-14) for the registrant and have:
a) designed such disclosure controls and procedures to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this annual report is being prepared;
b) evaluated the effectiveness of the registrant's disclosure controls and procedures as of a date within 90 days prior to the filing date of this annual report (the "Evaluation Date"); and
c) presented in this annual report our conclusions about the effectiveness of the disclosure controls and procedures based on our evaluation as of the Evaluation Date;
5. The registrant's other certifying officers and I have disclosed, based on our most recent evaluation, to the registrant's auditors and the audit committee of registrant's board of directors (or persons performing the equivalent functions):
a) all significant deficiencies in the design or operation of internal controls which could adversely affect the registrant's ability to record, process, summarize and report financial data and have identified for the registrant's auditors any material weaknesses in internal controls; and
b) any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal controls; and
6. The registrant's other certifying officers and I have indicated in this annual report whether there were significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of our most recent evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses.
Date: May 8, 2003
| /s/ JAMES M. GOWER James M. Gower Chairman and Chief Executive Officer |
75
CERTIFICATION
I, James H. Welch, certify that:
1. I have reviewed this annual report on Form 10-K/A of Rigel Pharmaceuticals, Inc.;
2. Based on my knowledge, this annual report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this annual report;
3. Based on my knowledge, the financial statements, and other financial information included in this annual report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this annual report;
4. The registrant's other certifying officers and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-14 and 15d-14) for the registrant and have:
a) designed such disclosure controls and procedures to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this annual report is being prepared;
b) evaluated the effectiveness of the registrant's disclosure controls and procedures as of a date within 90 days prior to the filing date of this annual report (the "Evaluation Date"); and
c) presented in this annual report our conclusions about the effectiveness of the disclosure controls and procedures based on our evaluation as of the Evaluation Date;
5. The registrant's other certifying officers and I have disclosed, based on our most recent evaluation, to the registrant's auditors and the audit committee of registrant's board of directors (or persons performing the equivalent functions):
a) all significant deficiencies in the design or operation of internal controls which could adversely affect the registrant's ability to record, process, summarize and report financial data and have identified for the registrant's auditors any material weaknesses in internal controls; and
b) any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal controls; and
6. The registrant's other certifying officers and I have indicated in this annual report whether there were significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of our most recent evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses.
Date: May 8, 2003
| /s/ JAMES H. WELCH James H. Welch Vice President, Chief Financial Officer and Secretary |
76
| 3.1(1) | Amended and Restated Certificate of Incorporation. | |
| 3.2(1) | Amended and Restated Bylaws. | |
| 4.1(1) | Specimen Common Stock Certificate. | |
| 4.2(1) | Amended and Restated Investor Rights Agreement, dated February 3, 2000, between Rigel and holders of Rigel's Series B, Series C, Series D and Series E preferred stock. | |
| 4.3(1) | Form of warrant to purchase shares of common stock. | |
| 4.7(11) | Amended and Restated Warrant issued to Kwacker Limited for the purchase of shares of common stock. | |
| 4.8(7) | Warrant issued to TBCC Funding Trust II for the purchase of shares of Common Stock. | |
| 4.9(8) | Warrant issued to Comerica Bank-California for the purchase of shares of Common Stock | |
| 4.10(11) | Warrant issued to Kwacker Limited for the purchase of shares of common stock. | |
| 4.11(11) | Warrant issued to Lighthouse Capital Partners IV, L.P. to purchase shares of common stock. | |
| 10.1(1) | Form of Indemnity Agreement. | |
| 10.2(11)(2) | 2000 Equity Incentive Plan, as amended. | |
| 10.3(1)(2) | Form of Stock Option Agreement pursuant to 2000 Equity Incentive Plan. | |
| 10.4(1)(2) | 2000 Employee Stock Purchase Plan. | |
| 10.5(1)(2) | 2000 Non-Employee Directors' Stock Option Plan. | |
| 10.6(1) | Collaboration Agreement between Rigel and Janssen Pharmaceutica N.V., dated December 4, 1998. | |
| 10.7(1) | Collaborative Research and License Agreement between Rigel and Pfizer Inc., dated January 31, 1999. | |
| 10.8(1) | Collaboration Agreement between Rigel and Novartis Pharma AG, dated May 26, 1999. | |
| 10.9(1)(3) | License and Research Agreement between Rigel and Cell Genesys, Inc., dated September 2, 1999. | |
| 10.10(1) | Collaborative Research and Development Agreement between Rigel and Neurocrine Biosciences, Inc., dated December 1997. | |
| 10.11(1)(2) | Employment Agreement between Rigel and Donald Payan, dated January 16, 1997. | |
| 10.12(1) | Lease between Rigel and Britannia Pointe Grand Limited Partnership, dated June 2, 1998. | |
| 10.13(1) | Technology Transfer Agreement between Rigel and Questcor Pharmaceuticals, Inc., dated September 22, 2000. | |
| 10.14(3)(4) | License and Research Agreement (Amended and Restated) between Rigel and Cell Genesys, Inc., dated September 2, 1999, as amended and restated on March 26, 2001. | |
| 10.15(5) | Lease termination agreement between Rigel and Britannia Pointe Grand Limited Partnership, dated May 6, 2001. | |
| 10.16(5) | Build-to-suit lease between Rigel and Slough BTC, LLC, dated May 16, 2001. | |
| 10.17(5) | First amendment to the Collaboration Agreement between Rigel and Novartis Pharma AG, dated May 18, 2001. | |
| 10.18(3)(6) | Second Amendment, dated July 6, 2001, to the Collaboration Agreement between Rigel and Novartis Pharma AG. | |
| 10.19(3)(6) | Second Amendment, dated July 1, 2001, to the Collaboration Agreement between Rigel and Cell Genesys, Inc. | |
| 10.20(2)(11) | 2001 Non-Officer Equity Incentive Plan, as amended. | |
| 10.21(2)(7) | Form of Stock Option Agreement pursuant to the 2001 Non-Officer Equity Incentive Plan. | |
| 10.22(8) | First Amendment, dated June 30, 2000, to the Collaboration Agreement by and between Rigel and Janssen Pharmaceutical N.V. | |
| 10.23(8) | Second Amendment, dated December 4, 2001, to the Collaboration Agreement by and between Rigel and Janssen Pharmaceutical N.V. | |
| 10.24(10) | Loan and Security Agreement between Rigel and Comerica Bank—California, dated July 12, 2002. | |
| 10.25(10)(3) | Collaboration Agreement between Rigel and Daiichi Pharmaceutical Co., Ltd., dated August 1, 2002. | |
| 10.26(11)(3) | Amendment to Build-to-suit lease between Rigel and Slough BTC, LLC, dated October 18, 2002. | |
| 10.27(11) | Master Lease Agreement between Rigel and Lighthouse Capital Partners IV, L.P., dated December 23, 2002. | |
| 10.28(2)(12) | Employment Agreement between Rigel and Elliott B. Grossbard, dated as of March 18, 2002. | |
| 10.29(2)(12) | Amendment to Employment Agreement, dated as of March 5, 2003, between Rigel and Donald Payan. | |
| 23.1(12) | Consent of Ernst & Young LLP, Independent Auditors. | |
| 24.1(11) | Power of Attorney. | |
| 99.1(12)(13) | Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |