UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| (Mark One) | |
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2003 |
|
or |
|
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number 0-29889
RIGEL PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
94-3248524 (IRS Employer Identification Number) |
|
1180 Veterans Blvd. South San Francisco, California (Address of principal executive offices) |
94080 (Zip Code) |
|
(650) 624-1100 (Registrant's telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: None Securities registered pursuant to Section 12(g) of the Act: Common Stock, par value $0.001 per share (Title of Class) |
||
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by a check mark whether the registrant is an accelerated filer (as defined in Exchange Act Rule 12b-2) Yes ý No o
The approximate aggregate market value of the Common Stock held by non-affiliates of the registrant, based upon the closing price of the registrant's Common Stock as reported on the Nasdaq National Market on June 30, 2003, the last business day of the registrant's most recently completed second fiscal quarter, was $50,050,000. The determination of affiliate status for the purposes of this calculation is not necessarily a conclusive determination for other purposes.
As of February 27, 2004, there were 17,903,522 shares of the registrant's Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Items 10, 11, 12, 13 and 14 of Part III incorporate information by reference from the definitive proxy statement for the Registrant's Annual Meeting of Stockholders to be held on June 10, 2004.
| |
|
Page |
|||
|---|---|---|---|---|---|
| PART I | |||||
Item 1. |
Business |
1 |
|||
Item 2. |
Properties |
25 |
|||
Item 3. |
Legal Proceedings |
25 |
|||
Item 4. |
Submission of Matters to a Vote of Security Holders |
25 |
|||
PART II |
|||||
Item 5. |
Market for the Registrant's Common Equity and Related Stockholder Matters |
26 |
|||
Item 6. |
Selected Financial Data |
27 |
|||
Item 7. |
Management's Discussion and Analysis of Financial Condition and Results of Operations |
28 |
|||
Item 7A. |
Quantitative and Qualitative Disclosures About Market Risk |
37 |
|||
Item 8. |
Financial Statements and Supplementary Data |
39 |
|||
Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
60 |
|||
Item 9A. |
Controls and Procedures |
60 |
|||
PART III |
|||||
Item 10. |
Directors and Executive Officers of the Registrant |
62 |
|||
Item 11. |
Executive Compensation |
62 |
|||
Item 12. |
Security Ownership of Certain Beneficial Owners and Management |
62 |
|||
Item 13. |
Certain Relationships and Related Transactions |
62 |
|||
Item 14. |
Principal Accounting Fees and Services |
62 |
|||
PART IV |
|||||
Item 15. |
Exhibits, Financial Statement Schedules and Reports on Form 8-K |
62 |
|||
Signatures |
66 |
||||
Item 1. Business
This annual report on Form 10-K, including the documents that we incorporate by reference, contains statements indicating expectations about future performance and other forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that involve risks and uncertainties. We usually use words such as "may," "will," "should," "expect," "plan," "anticipate," "believe," "estimate," "predict," "future," "intend," "potential" or "continue" or the negative of these terms or similar expressions to identify forward-looking statements. These statements appear throughout this annual report on Form 10-K and are statements regarding our current intent, belief or expectation, primarily with respect to our operations and related industry developments. Examples of these statements include, but are not limited to, statements regarding the following: our business and scientific strategies; the progress of our product development programs, including clinical testing; our corporate collaborations, including revenues received from these collaborations; our drug discovery technologies; our research and development expenses; protection of our intellectual property; sufficiency of our cash resources; and our operations and legal risks. You should not place undue reliance on these forward-looking statements, which apply only as of the date of this annual report on Form 10-K. Our actual results could differ materially from those anticipated in these forward-looking statements for many reasons. Any forward-looking statement speaks only as of the date on which it is made, and we undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
Overview
Rigel's mission is to become a source of novel, small-molecule drugs to meet large, unmet medical needs. We have three initial development programs: allergy/asthma, hepatitis C and rheumatoid arthritis. We have begun clinical testing of our first two product candidates, R112 for allergic rhinitis and R803 for hepatitis C, and plan to begin clinical trials of two additional product candidates, for the treatment of rheumatoid arthritis and asthma, by the end of 2004. We own the economic and commercial rights to these product candidates. Our business model is to develop a portfolio of product candidates and to take these product candidates through Phase II clinical trials, after which we intend to seek partners for completion of clinical trials, regulatory approval and marketing. Our approach to drug discovery is based on advanced, proprietary techniques that allow us to identify targets with a demonstrable role in a disease pathway and to screen efficiently for those targets that are likely to be amenable to drug modulation. We believe that this approach to drug discovery will enable us to commence clinical trials with one to two lead compounds each year. Our research efforts are focused in the areas of immunology/inflammation, virology and oncology. We were incorporated in Delaware in June 1996, and we are based in South San Francisco, California.
Our Strategy
Our strategy is to develop a portfolio of product candidates that can be developed into small molecule therapeutics. We believe that producing a portfolio of many product candidates and working in conjunction with pharmaceutical companies to further develop those candidates increases our probability of commercial success. By utilizing our technology to rapidly discover and validate new targets and product candidates in a wide range of applications, we can generate a portfolio of potential product candidates. We believe that our portfolio approach allows us to minimize the risk of failure by
1
pursuing many product candidates at once, while concurrently being well positioned to help fill a continuing product pipeline gap at major pharmaceutical companies.
The product development process is one that is subject to both high costs and high risk of failure. We intend to identify a portfolio of new product candidates across a broad range of diseases and develop them through Phase II clinical trials. Rather than incur the costs of taking product candidates all the way through the drug approval process and exposing ourselves to the risk of failure associated with Phase III clinical trials, we intend to partner with pharmaceutical and biotechnology companies when the costs and risks associated with Phase III clinical trials are too great for us to pursue approval independently. We believe that multiple product candidates can be developed through Phase II clinical trials for approximately the same cost as would be required to take one product candidate through Phase III clinical trials and marketing approval. The key elements of our scientific and business strategy are to:
Clinical and Preclinical Product Development Programs
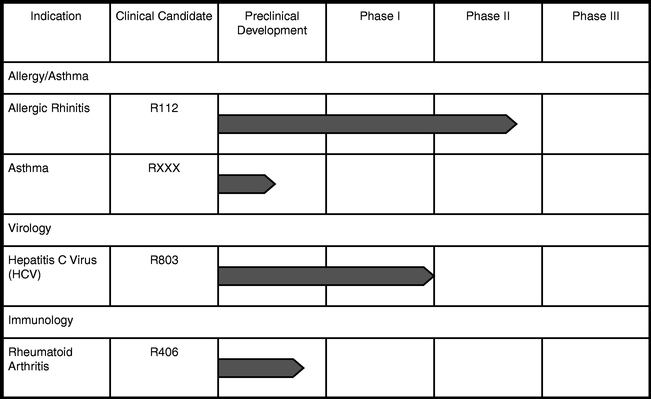
We conduct research programs for our own proprietary programs. We are developing several proprietary product candidates. Our most advanced development efforts are described below. The following table summarizes the current status of our proprietary clinical development programs by specific disease mechanisms:
These Programs are:

2
Allergy/Asthma
Disease background. Allergic rhinitis and asthma are chronic inflammatory disorders of the airways. Allergic rhinitis, or allergy, is an acute inflammatory reaction in the upper respiratory tract resulting in nasal congestion, sneezing, itching and watery eyes. Asthma effects the lower respiratory tract and is marked by episodic flare-ups, or attacks, that can be life threatening. In some patients, allergens, such as pollen, trigger the production of immunoglobulin E antibodies, or IgE antibodies, which then bind to mast cells and cause an intracellular signal that results in the release of various chemical mediators. When this process occurs repeatedly over time, it creates persistent inflammation of the airway passages, resulting in the chronic congestion and airway obstruction associated with allergic rhinitis and asthma, respectively. Over 59 million people in the United States suffer from allergic disorders, and over 11 million people suffer from asthmatic disorders.
Allergic rhinitis program. Our first clinical candidate, R112, is an intranasal inhibitor to Syk, or spleen tyrosine kinase, a novel drug target for respiratory diseases such as allergic rhinitis and asthma. Syk is involved in IgE signaling in mast cells. Mast cells play important roles in both early and late phase allergic reactions, and Syk inhibitors could prevent both phases. We completed a Phase I clinical trial of R112 in 18 patients in December 2002, a single-dose Phase I/II clinical trial of 20 patients in June 2003 and a multi-dose safety trial of 24 patients in December 2003.
The single-dose Phase I/II clinical trial evaluated the efficacy and safety of a single intranasal administration of R112 in volunteer patients with asymptomatic seasonal allergic rhinitis. The preliminary results of this study indicate that R112 was well tolerated. In addition, R112 demonstrated physiological responses, including significant statistical improvement or consistent positive trends in reducing the release of chemical mediators involved in mast cell activation, one of the earliest steps in the initiation of an inflammatory response in allergy and asthma. The multi-dose safety trial results indicated that R112 is well tolerated and demonstrates a favorable safety profile in the study population. Specifically, the key findings of this study include no local nasal irritation due to the administration of R112 and no significant laboratory abnormalities.
Based on the results of the single and multi-dose trials, we plan to initiate in the first half of 2004 Phase II clinical trials that will measure allergic symptom improvement and treatment. This randomized, placebo-controlled park study will take place in two locations in different parts of the country where patients will spend two days in an outdoor setting during the high-pollen season. We expect to receive the results of this trial in the second half of 2004.
Asthma program. We are currently working on next generation, inhaled and oral Syk inhibitors to address asthma. The selection and clinical program for this indication may be influenced by the possible execution of a strategic partnership in the area of allergy/asthma. We expect to choose a lead candidate to move forward into the clinic later in 2004 and anticipate initiating clinical trials in asthma late in 2004.
3
Hepatitis C Virus
Disease and current treatment approaches. Hepatitis C is an inflammation of the liver caused by the hepatitis C virus. As the most common chronic blood-borne infection in the United States, the hepatitis C virus, or HCV, affects an estimated 3.9 million people in the United States and 170 million individuals worldwide. Approximately 80 percent of those with acute illness will develop chronic hepatitis, a condition that has been linked to cirrhosis, liver failure and hepatocellular carcinoma, or liver cancer. HCV is a leading cause of chronic liver disease and is the most common indication for liver transplantation.
Currently available HCV therapies are only modestly effective at treating the disease. The most prevalent treatment regimen is with interferon alpha, or IFN, or its longer lasting pegylated version, usually in combination with ribavarin. IFN therapy works to boost the body's own immune system and generally requires six to 12 months of therapy to be effective. Only 20 percent to approximately 40 percent of the patients who complete IFN therapy have a successful response. IFN dosage must be reduced in 10 percent to 40 percent of patients and discontinued in 5 percent to 15 percent of patients because of severe side effects. Moreover, IFN is least effective against HCV genotype 1, the strain responsible for approximately 70 percent of chronic HCV cases in the United States.
Anti-HCV program. Our lead anti-HCV compound, R803, is an oral, small molecule that, in our preclinical studies, works directly, rapidly and selectively on the virus by interfering with a viral polymerase protein that is needed for replication. To date, R803 has demonstrated potent activity in inhibiting viral replication in preclinical experiments. In various laboratory experiments, R803 appears to act within days to reduce viral levels, and has been shown to be active against various genotypes of HCV, including genotype 1.
We completed our initial Phase I clinical trial of R803 in January 2004. Clinical data indicates that R803 is well tolerated with no clinically significant adverse effects reported in the dosing schedule that we plan to use in further clinical trials. In the Phase I clinical trial, an escalating dose regimen of R803 was studied in 34 volunteers and was compared with 8 volunteers who received a placebo. The trial was conducted in the United Kingdom, and the results will be part of the U.S. IND package that we expect to file with the Food and Drug Administration, or FDA, in the first quarter of 2004.
We plan to commence a Phase I/II clinical trial of R803 in the United States during the second quarter of 2004 in HCV-infected patients. This trial will monitor HCV viral levels and safety over numerous days of drug administration.
Rheumatoid Arthritis
Disease and current therapeutic approaches. Rheumatoid arthritis is a chronic inflammatory disease affecting multiple tissues, but typically producing its most pronounced symptoms in the joints. It is progressive, degenerative and ultimately debilitating. The chronic inflammation in joints leads to the destruction of the soft tissue—the synovium and cartilage—as well as to erosion of the articular surfaces of bones. The disease is estimated to affect over 2 million people in the United States. It is more prevalent in women, who are estimated to account for 1.5 million of the cases.
Currently, rheumatoid arthritis is not well treated, with most therapies having significant potential side effects or other shortfalls. Rheumatoid arthritis patients receive multiple drugs depending on the extent and aggressiveness of the disease. Initially, patients receive a non-steroidal anti-inflammatory, or a NSAID, or a Cox-2 inhibitor, another anti-inflammatory drug. These drugs address the symptoms of rheumatoid arthritis, but not the underlying progressive destruction of bone and cartilage. As the disease progresses, NSAIDs are supplemented with steroids and then a disease-modifying anti-rheumatic drug, or DMARD, such as methotrexate, an anti-cancer agent, or an anti-TNF agent, such as Enbrel®. These latter drugs block only the inflammatory mediator, TNF, and are all delivered
4
via injection. The side effects (in the case of methotrexate), and delivery (in the case of the anti-TNF agents), limit their use to late in the course of the disease after significant bone and cartilage damage has already occurred.
Rheumatoid arthritis program. We have selected R406 as our lead product candidate for initial clinical trials in rheumatoid arthritis. R406 is a novel, oral syk kinase inhibitor that, in preclinical studies, blocks the activation of mast cells and B cells that promote the swelling and inflammatory response. R406 has been shown effective in preliminary animal models of arthritis and appears to be well tolerated in preclinical studies. Data from preclinical studies indicate that R406 is effective at low doses in a rodent arthritis model, and was without obvious toxicities in the same model at doses well above the effective dose. We expect to file an IND application with the FDA for the indication of rheumatoid arthritis in the second half of 2004.
Proprietary Research Programs
We are conducting proprietary research in three broad disease areas: immunology/inflammation, virology and oncology. With each disease area we are conducting basic research as well as screening compounds against potential novel intracellular targets and optimizing those leads which appear most promising.
We are researching autoimmune mediated inflammation disorders such as multiple sclerosis and inflammation of the bowel. We have identified more than one kinase that may be inhibited in order to treat inflammation related disorders, and we are in the process of screening other compounds against various kinases in order to find additional lead compounds to potentially treat inflammation related disorders. In the area of virology, we are investigating other potential targets to inhibit HCV replication. In addition, we are conducting initial screening tests of potential product candidates against other viruses. In the area of oncology, we are focused on inhibiting kinases as well as ligases, a new target class which also may yield possible drug targets in the immunology and virology areas.
Corporate Collaborations
Current Collaborations
In addition to the preceding programs in which we retain all commercial and economic rights, we also carry on research and development programs in connection with our corporate collaborations. We currently have collaborations with four major pharmaceutical companies, including one with Janssen Pharmaceutica N.V., a division of Johnson & Johnson, relating to oncology therapeutics and diagnostics, one with Pfizer Inc. relating to asthma and allergy therapeutics, one with Novartis Pharma AG with four different programs relating to immunology, oncology and chronic bronchitis and one with Daiichi Pharmaceuticals Co., Ltd. in the area of oncology. These collaborations all have or had a research phase during which we receive or received funding based on the level of headcount allocated to a program. After the research phase concludes, we are entitled to certain milestones and royalties. Currently, only the Novartis oncology and chronic bronchitis programs and the Daiichi program are in the research phase of the agreements. In addition, we have a number of scientific collaborations with academic institutions and biotechnology companies under which we have in-licensed technology.
Johnson & Johnson
Effective December 1998, we entered into a three-year research collaboration, which was extended through December 2003, with Johnson & Johnson, to identify, discover and validate novel drug targets that regulate cell cycle, and, specifically, to identify drug targets and the active peptides that bind to them that can restore a mutated cell's ability to stop uncontrolled cell division. Under the agreement, we are providing certain assays and associated technology to Johnson & Johnson for the assessment of the alteration or normalization of the dysfunctional cell cycles of cancer cells for Johnson & Johnson's
5
internal research purposes. Furthermore, in an amendment to the collaboration in July 2000, Johnson & Johnson expanded the collaboration whereby we performed compound screening and medicinal chemistry on some of the validated targets accepted by Johnson & Johnson. We have identified several novel drug targets in this program, four of which have been accepted by Johnson & Johnson as validated. Two of these four targets have completed high-throughput screening, or HTS, at Rigel. Johnson & Johnson is obligated to pay us various milestones and royalties if certain conditions are met.
Pfizer
Effective January 1999, we entered into a research collaboration with Pfizer to identify and validate intracellular drug targets that control and inhibit the production of IgE in B Cells in the area of asthma/allergy. The research phase of the collaboration was initially scheduled to end on January 31, 2001. In January 2001, Pfizer notified us of its election to exercise its option to extend the funded research portion of the collaboration one additional year to January 31, 2002. During the research phase at Rigel, the collaboration was successful in identifying several intracellular drug targets that control the production of IgE, a key mediator in allergic reactions and asthma in B cells. Through the conclusion of the research phase of the collaboration, which was extended by one additional month to February 28, 2002, Pfizer accepted a total of seven validated targets. Pfizer is obligated to pay us various milestones and royalties if certain conditions are met.
Novartis
In May 1999, we signed an agreement for the establishment of a broad collaboration with Novartis. We agreed to work with Novartis on up to five different five-year research projects to identify drug targets for products that can treat, prevent or diagnose the effects of human disease. Two of the research projects would be conducted jointly by Novartis and us, and the other three research projects were to be conducted at Novartis. The first research project, a joint research project, was focused on identifying small molecule drug targets that regulate T cells in the area of transplant rejection. The second research project, also a joint research project, related to the identification and validation of small molecule drug targets that mediate specific functions of B cells in the area of autoimmunity. Pursuant to the collaboration agreement, Novartis had the option to end the research phase on these programs after either 24 months or 42 months. In May 2002, Novartis elected to conclude the research phases of our two initial joint projects in the autoimmunity and transplant rejection areas, after 42 months each, effective in November 2002 and February 2003, respectively. The third research project, a project currently being carried out at Novartis, is focused on identifying small molecule drug targets that regulate chronic bronchitis. Novartis may terminate this chronic bronchitis research at any time. In July 2001, we amended the agreement to add a three-year joint project at Rigel in the area of angiogenesis in lieu of a project at Novartis. This resulted in both funded research at Rigel and an additional upfront payment of $4.0 million, which were terms not previously included in the project at Novartis. In January 2002, Novartis chose not to exercise its option to add a second project to be conducted at Novartis. Novartis is obligated to pay us various milestones and royalties if certain conditions are met.
Daiichi
In August 2002, we signed an agreement for the establishment of a collaboration with Daiichi to pursue research related to a specific target from a novel class of drug targets called ligases that control cancer cell proliferation through protein degradation. Per the agreement, the research phase of this collaboration is for three years. We are working with Daiichi to discover and develop cancer pharmaceutical drugs. Under the terms of the collaboration agreement, Daiichi has paid us $0.9 million upfront, two milestone payments totaling $3.7 million, is obligated to pay us ongoing research support
6
through August 2005 and may become obligated to pay us certain other milestones payments. In addition, we are entitled to receive royalties on any commercialized products to emerge from the collaboration.
The initial stages of the Daiichi collaboration focused on the development of the assay for the specific target and the initiation of high-throughput compound screening to identify therapeutic molecules we and Daiichi would like to advance to later stages of product development. Under terms of the agreement, we retain the rights to co-develop and co-promote products resulting from this collaboration in North America while Daiichi retains co-development and promotion rights in the remainder of the world.
Future Collaborations
We are exploring new opportunities with existing and new potential collaborators. Our earliest partnerships focused on the early stages of drug discovery, specifically on target discovery and validation, while our collaboration with Johnson & Johnson has been expanded to also include both chemistry and compound HTS. Our recent collaboration with Daiichi focuses on drug discovery and development. We currently anticipate that in order to support our current research programs we will need to self-fund, at an increased rate of spending, our own research programs to later stages of development prior to partnering with collaborative partners. Therefore, it is expected that future collaborative partnerships may have an expanded focus and could include HTS, combinatorial and medicinal chemistry, preclinical evaluations and/or clinical development. For some programs, we may also seek to enter into collaborations for the development of compounds that we have discovered. For example, we have received preliminary human efficacy data for our lead compound R112 for the treatment of allergic rhinitis. We expect that this program could be the basis of our next corporate collaboration, which we anticipate entering into in 2004.
Our Solution
The technologies that we use in connection with both our proprietary product development programs and our corporate collaborations are designed to identify protein targets for compound screening and validate the role of those targets in the disease process. Unlike genomics-based approaches, which begin by identifying genes and then search for their functions, our approach identifies proteins that are demonstrated to have an important role in a disease pathway. By understanding the disease pathway, we attempt to avoid studying genes that will not make good drug targets and focus only on the sub-set of expressed proteins of genes that we believe are specifically implicated in the disease process.
We begin by developing assays that model the key events in a disease process at the cellular level. We then efficiently search hundreds of millions of cells to identify potential protein targets. In addition, we identify the proteins involved in the intracellular process and prepare a map of their interactions, thus giving us a comprehensive picture of the intracellular disease pathway. We believe that our approach has a number of advantages, including:
7
Because of the very large number of cells and proteins employed, our technology is labor intensive. The complexity of our technology requires a high degree of skill and diligence to perform successfully. In addition, successful application of our technology depends on a highly diverse collection of proteins to test in cells. We believe we have been able to and will continue to meet these challenges successfully. Although other companies may utilize technologies similar to certain aspects of our technology, we are unaware of any other company that employs the same combination of technologies as we do.
Technology
Our retroviral and pathway mapping technologies enable us to identify and validate new protein targets and establish a map of the intracellular proteins that define a specific signaling pathway controlling cellular responses. We believe that, together, these technologies allow for rapid pathway mapping of complex biological processes and increase our ability to identify targets for drug discovery.
Retroviral Functional Screening.
Our retroviral technology introduces up to 100 million different peptides, or proteins, into an equal number of normal or diseased cells. Each retrovirus delivers a specific gene into an individual cell, causing the cell to produce a specific protein. Then, we stimulate the cells in a manner known to produce a disease-like behavioral response or phenotype of the disease process. Once in the cell, the expressed protein interacts with potential protein targets in the cell. Then, we sort the cells at a rate of up to 60,000 cells/second to collect data on up to five different parameters, which means that a sort of 100 million cells can be completed in approximately half an hour. By analyzing the approximately 500 million resulting data points, we can rapidly identify those few cells containing an expressed protein that has interacted with a protein target in a way that causes the cell to change its behavior from diseased back to normal. Using this method, we believe that we can identify the relatively few targets that are validated in the context of a disease-specific cellular response.
Pathway Mapping.
Our pathway mapping technology identifies specific proteins that bind with other proteins that are known to be part of a signaling pathway, either because we identified them using our retroviral technology or because the proteins have been described in the scientific literature. This pathway mapping technology is directed at:
8
Using our pathway mapping technology, we split a protein that gives a detectable signal (reporter protein), such as fluorescence, into two inactive parts. One part of the reporter protein is fused with a specific protein known to be involved in a signaling disease-relevant pathway (bait protein). Multiple copies of the other part of the reporter protein are fused one by one with all the proteins known to be present in the cell type being studied (library protein). When the bait protein binds to a specific library protein, the two parts of the reporter protein reunite and become active again, thereby generating a detectable signal. We employ an improved version of the two hybrid protein interaction method in yeast cells. In addition, we have developed a patented method of employing the two hybrid protein interaction technology in mammalian cells. Mammalian cells offer the opportunity to monitor protein-protein interactions in a potentially more relevant cellular environment.
We also use this pathway mapping technology to screen identified protein targets against a library of peptides in order to identify each active interaction site on the target. This information is useful in directing our chemistry efforts to identify compounds specifically designed to bind to the interaction site on the target.
Target Validation
The first step of our target validation occurs when we use our retroviral technology to identify targets. We design a screen that reflects a key event in a disease process so that when one of our proteins changes the behavior of a specific cell, this indicates a causal relationship between the protein-target interaction and the specific disease response. This approach saves time and enhances the probability that those targets that are identified and pursued are disease relevant. It also tells us that the protein interacts with a functional site on the target since the interaction results in a change in the behavior of the cell. We further validate the function of specific targets by:
Other Technologies
Our drug discovery technologies utilize the following additional technologies:
High-Throughput Compound Screening
Using our cell sorter system, we conduct screening of small molecule compounds in the same cell-based disease-specific screens that we use to identify the protein targets. This enables us to screen thousands of compounds in a matter of a few hours, while simultaneously examining multiple physiological parameters. In addition, we have established conventional high-throughput screens of small molecule compounds using biochemical methods similar to those widely used in the biotechnology and pharmaceutical industries. We have a library of approximately 220,000 small molecule compounds having highly diverse molecular structures for our compound screening activities.
We select for compound screening only those protein drug targets we judge to meet several criteria:
9
Medicinal and Combinatorial Chemistries
Our medicinal chemistry group carries out traditional structure-activity relationship studies of potential lead compounds and makes improvements to those compounds by utilizing chemistry techniques to synthesize new analogs of a lead compound with improved properties. Our chemistry group synthesizes compounds incorporating desirable molecular features. We also utilize outside contract research organizations from time to time to supplement our internal chemistry resources.
Pharmacology and Preclinical Development
We believe that the rapid characterization and optimization of lead compounds identified in HTS will generate high-quality preclinical development candidates. Our pharmacology and preclinical development group facilitates lead optimization by characterizing lead compounds with respect to pharmacokinetics, potency, efficacy and selectivity. The generation of proof-of-principle data in animals and the establishment of standard pharmacological models with which to assess lead compounds represent integral components of lead optimization. As programs move through the lead optimization stage, our pharmacology and preclinical development group supports our chemists and biologists by performing the necessary studies, including toxicology, for IND application submissions.
Clinical Development
We have assembled a team of experts in drug development to design and implement clinical trials and to analyze the data derived from these studies. The clinical development group possesses expertise in project management and regulatory affairs.
Research and Development Expenses
Our research and development expenses were $43.4 million in both 2003 and 2002 and $32.3 million in 2001.
Intellectual Property
We will be able to protect our technology from unauthorized use by third parties only to the extent that it is covered by valid and enforceable patents or is effectively maintained as trade secret. Accordingly, patents or other proprietary rights are an essential element of our business. We have over 135 pending patent applications and 39 issued patents in the United States that are owned or exclusively licensed in our field as well as pending corresponding foreign patent applications. Our policy is to file patent applications to protect technology, inventions and improvements to inventions that are commercially important to the development of our business. We seek United States and international patent protection for a variety of technologies, including new screening methodologies and other research tools, target molecules that are associated with disease states identified in our screens, and lead compounds that can affect disease pathways. We also intend to seek patent protection or rely upon trade secret rights to protect other technologies that may be used to discover and validate targets and that may be used to identify and develop novel drugs. We seek protection, in part, through confidentiality and proprietary information agreements. We are a party to various other license agreements that give us rights to use technologies in our research and development.
10
In June 2002, we resolved a dispute with Inoxell A/S (formed as a spinout from Pharmexa—formally M&E Biotech) by entering into a global patent settlement concerning certain drug target identification technologies, which includes both cross-licensing and joint ownership to certain patents and allows for worldwide freedom of operation for both companies. Originally, Inoxell notified us that it had received patent protection in some European countries and Australia for a process that it asserted was similar to certain aspects of our technologies.
Competition
We face, and will continue to face, intense competition from pharmaceutical and biotechnology companies, as well as from academic and research institutions and government agencies, both in the United States and abroad. Some of these competitors are pursuing the development of pharmaceuticals that target the same diseases and conditions as our research programs. Our major competitors include fully integrated pharmaceutical companies that have extensive drug discovery efforts and are developing novel small molecule pharmaceuticals. We also face significant competition from organizations that are pursuing the same or similar technologies, including the discovery of targets that are useful in compound screening, as the technologies used by us in our drug discovery efforts. Our competitors or their collaborative partners may utilize discovery technologies and techniques or partner more rapidly or successfully than we or our collaborators are able to do.
Many of these companies and institutions, either alone or together with their collaborative partners, have substantially greater financial resources and larger research and development staffs than we do. In addition, many of these competitors, either alone or together with their collaborative partners, have significantly greater experience than we do in:
Accordingly, our competitors may succeed in obtaining patent protection, identifying or validating new targets or discovering new drug compounds before we do.
Competition may also arise from:
Developments by others may render our product candidates or technologies obsolete or noncompetitive. We face and will continue to face intense competition from other companies for collaborative arrangements with pharmaceutical and biotechnology companies, for establishing relationships with academic and research institutions and for licenses to additional technologies. These competitors, either alone or with their collaborative partners, may succeed in developing technologies or products that are more effective than ours.
Our ability to compete successfully will depend, in part, on our ability to:
11
Government Regulation
Our ongoing development activities are and will be subject to extensive regulation by numerous governmental authorities in the United States and other countries, including the FDA under the Federal Food, Drug and Cosmetic Act. The regulatory review and approval process is expensive and uncertain. Securing FDA approval requires the submission of extensive preclinical and clinical data and supporting information to the FDA for each indication to establish a product candidate's safety and efficacy. The approval process takes many years, requires the expenditure of substantial resources and may involve ongoing requirements for post-marketing studies. Clinical trials are subject to oversight by institutional review boards and the FDA and:
Even if we are able to achieve success in our clinical testing, we, or our collaborative partners, must provide the FDA and foreign regulatory authorities with clinical data that demonstrates the safety and efficacy of our products in humans before they can be approved for commercial sale. We also do not know whether any future clinical trials will demonstrate sufficient safety and efficacy necessary to obtain the requisite regulatory approvals or will result in marketable products. Our failure, or the failure of our strategic partners, to adequately demonstrate the safety and efficacy of our products under development will prevent receipt of FDA and similar foreign regulatory approval and, ultimately, commercialization of our products.
Any clinical trial may fail to produce results satisfactory to the FDA. Preclinical and clinical data can be interpreted in different ways, which could delay, limit or prevent regulatory approval. Negative or inconclusive results or adverse medical events during a clinical trial could cause a clinical trial to be repeated or a program to be terminated. In addition, delays or rejections may be encountered based upon additional government regulation from future legislation or administrative action or changes in FDA policy or interpretation during the period of product development, clinical trials and FDA regulatory review. Failure to comply with applicable FDA or other applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production or injunction, as well as other regulatory action against our potential products, collaborative partners or us. Additionally, we have no experience in working with our partners in conducting and managing the clinical trials necessary to obtain regulatory approval.
Outside the United States, our ability to market a product is contingent upon receiving a marketing authorization from the appropriate regulatory authorities. The requirements governing the conduct of clinical trials, marketing authorization, pricing and reimbursement vary widely from country to country. At present, foreign marketing authorizations are applied for at a national level, although
12
within the European Union, or EU, registration procedures are available to companies wishing to market a product in more than one EU member state. If the regulatory authority is satisfied that adequate evidence of safety, quality and efficacy has been presented, a marketing authorization will be granted. This foreign regulatory approval process involves all of the risks associated with FDA clearance.
Employees
As of December 31, 2003, we had 126 employees.
Scientific Advisors
We utilize scientists and physicians to advise us on scientific and medical matters as part of our ongoing research and product development efforts, including experts in human genetics, mouse genetics, molecular biology, biochemistry, cell biology, chemistry, infectious diseases, immunology and structural biology. Certain of our scientific and medical advisors and consultants receive an option to purchase our common stock and an honorarium for time spent assisting us.
Available Information
We maintain a site on the world wide web at www.rigel.com; however, information found on our website is not incorporated by reference into this annual report on Form 10-K. We file electronically with the Securities and Exchange Commission our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. We make available free of charge on or through our website copies of these reports as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission. Further, a copy of this annual report on Form 10-K is located at the Securities and Exchange Commission's Public Reference Room at 450 Fifth Street, NW, Washington, D.C. 20549. Information on the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330. The Securities and Exchange Commission maintains an internet site that contains reports, proxy and information statements and other information regarding our filings at www.sec.gov.
Risk Factors
An investment in our securities is risky. Prior to making a decision about investing in our securities you should carefully consider the following risks, as well as the other information contained in this annual report on Form 10-K. If any of the following risks actually occurs, our business could be harmed. In that case, the trading price of our securities could decline, and you might lose all or part of your investment. The risks and uncertainties described below are not the only ones facing us. Additional risks and uncertainties not presently known to us, or that we currently see as immaterial, may also harm our business. If any of these additional risks or uncertainties occur, the trading price of our securities could decline, and you might lose all or part of your investment.
We will need additional capital in the future to sufficiently fund our operations and research.
We have consumed substantial amounts of capital to date, and operating expenditures are expected to increase over the next several years. We believe that our existing capital resources, including the net proceeds to us from the recently completed public offering of our common stock and anticipated proceeds from current and future collaborations, will be sufficient to support our current operating plan through the second quarter of 2006. Our operations will require significant additional funding in large part due to our research and development expenses, future preclinical and clinical-testing costs, the expansion of our facilities and the absence of any meaningful revenues for the foreseeable future.
13
The amount of future funds needed will depend largely on the success of our collaborations and our research activities, and we do not know whether additional financing will be available when needed, or that, if available, we will obtain financing on terms favorable to our stockholders or us. We have consumed substantial amounts of capital to date, and operating expenditures are expected to increase over the next several years as we expand our infrastructure and research and development activities.
To the extent we raise additional capital by issuing equity securities, our stockholders would at that time experience substantial dilution. To the extent that we raise additional funds through collaboration and licensing arrangements, we may be required to relinquish some rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us.
Our future funding requirements will depend on many uncertain factors.
Our future funding requirements will depend upon many factors, including, but not limited to:
Insufficient funds may require us to delay, scale back or eliminate some or all of our research or development programs, to lose rights under existing licenses or to relinquish greater or all rights to product candidates at an earlier stage of development or on less favorable terms than we would otherwise choose or may adversely affect our ability to operate as a going concern.
Our success as a company is uncertain due to our limited operating history, our history of operating losses and the uncertainty of future profitability.
Due in large part to the significant research and development expenditures required to identify and validate new product candidates and pursue our development efforts, we have not been profitable and have generated operating losses since we were incorporated in June 1996. The extent of our future losses and the timing of potential profitability are highly uncertain, and we may never achieve profitable operations. We have incurred net losses of $41.2 million in 2003, $37.0 million in 2002 and $23.8 million in 2001. Currently, our revenues are generated solely from research payments from our collaboration agreements and licenses and are insufficient to generate profitable operations. We expect
14
that our future revenue from current collaborations will decline compared to previous periods. As of December 31, 2003, we had an accumulated deficit of approximately $156.0 million. We expect to incur losses for at least the next several years and expect that these losses will increase as we expand our research and development activities and incur significant clinical and testing costs.
There is a high risk that early-stage drug discovery and development might not successfully generate good product candidates.
At the present time, the majority of our operations are in the early stages of drug identification and development. To date, only two of our drug compounds have made it into the clinical testing stage. In our industry, it is statistically unlikely that the limited number of compounds that we have identified as potential product candidates will actually lead to successful product development efforts, and we do not expect any drugs resulting from our research to be commercially available for several years, if at all. Our two drug compounds in the clinic and our future leads for potential drug compounds will be subject to the risks and failures inherent in the development of pharmaceutical products based on new technologies. These risks include, but are not limited to, the inherent difficulty in selecting the right drug target and avoiding unwanted side effects as well as the unanticipated problems relating to product development, testing, regulatory compliance, manufacturing, marketing, competition and costs and expenses that may exceed current estimates. The results of preliminary studies do not necessarily predict clinical or commercial success, and larger later-stage clinical trials may fail to confirm the results observed in the preliminary studies. With respect to our own compounds in development, we have established anticipated timelines for clinical development based on existing knowledge of the compound. However, we cannot provide assurance that any specified timelines with respect to the initiation or completion of clinical studies may be achieved.
For example, we began a Phase I clinical trial of R112 in September 2002 in the United Kingdom. The data from this trial was incorporated into an IND application that was filed with the FDA in November 2002. Subsequently, we recently completed a Phase I/II clinical trial in which we evaluated the safety and effectiveness of R112 in patients with documented allergies. In addition, we recently completed a multi-dose safety trial of R112 with the goal of establishing the longer-term, multi-dose safety of R112 in various dosing regimens. Based on this study, we plan to initiate a Phase II clinical trial in early 2004. However, the timing of initiation of this study or the outcome cannot be predicted. We also recently completed a human safety trial in the United Kingdom of our compound, R803, for the treatment of hepatitis C. We plan to launch a Phase I/II clinical trial in the United States during the second quarter of 2004 in HCV-infected patients. Because of the uncertainty of whether the accumulated preclinical evidence (pharmacokinetic, pharmacodynamic, safety and/or other factors) or early clinical results will be observed in later clinical trials, we can make no assurance regarding the results likely from our future clinical trials or the impact of those results on our business.
We might not be able to commercialize our product candidates successfully if problems arise in the clinical testing and approval process.
Commercialization of our product candidates depends upon successful completion of preclinical studies and clinical trials. Preclinical testing and clinical development are long, expensive and uncertain processes. We do not know whether we, or any of our collaborative partners, will be permitted to undertake clinical trials of potential products beyond the trials already concluded and the trials currently in process. It may take us or our collaborative partners several years to complete any such testing, and failure can occur at any stage of testing. Interim results of trials do not necessarily predict final results, and acceptable results in early trials may not be repeated in later trials. A number of companies in the pharmaceutical industry, including biotechnology companies, have suffered significant setbacks in advanced clinical trials, even after achieving promising results in earlier trials. Moreover, as our projects reach clinical trials, we or our collaborative partners or regulators may decide to
15
discontinue development of any or all of these projects at any time for commercial, scientific or other reasons. For example, if patients experience undesirable side effects, we may be required to halt or suspend a clinical trial.
Delays in clinical testing could result in increased costs to us.
Significant delays in clinical testing could materially impact our product development costs. We do not know whether planned clinical trials will begin on time, will need to be revamped or will be completed on schedule, or at all. Clinical trials can be delayed for a variety of reasons, including delays in obtaining regulatory approval to commence a study, delays in reaching agreement on acceptable clinical study agreement terms with prospective clinical sites, delays in obtaining institutional review board approval to conduct a study at a prospective clinical site or delays in recruiting subjects to participate in a study. Environmental conditions may impact the execution of clinical trials, particularly in the allergy area.
In addition, we typically rely on third-party clinical investigators to conduct our clinical trials and other third-party organizations to oversee the operations of such trials and to perform data collection and analysis. As a result, we may face additional delaying factors outside our control if these parties do not perform their obligations in a timely fashion. While we have not yet experienced delays that have materially impacted our clinical trials or product development costs, delays of this sort could occur for the reasons identified above or other reasons. If we have delays in testing or approvals, our product development costs will increase. For example, we may need to make additional payments to third-party investigators and organizations to retain their services or we may need to pay recruitment incentives. If the delays are significant, our financial results and the commercial prospects for our product candidates will be harmed, and our ability to become profitable will be delayed.
We lack the capability to manufacture compounds for development and rely on third parties to manufacture our product candidates, and we may be unable to obtain required material in a timely manner, at an acceptable cost or at a quality level required to receive regulatory approval.
We currently do not have manufacturing capabilities or experience necessary to produce materials, including R112, R803 and R406, for preclinical testing and clinical trials. We rely on a single third-party contractor to produce R112, R803 and R406 bulk drug substance. We also rely on different single manufacturers for finished R112, R803 and R406 product for preclinical and clinical testing. We will rely on manufacturers to deliver materials on a timely basis and to comply with applicable regulatory requirements, including the FDA's current Good Manufacturing Practices, or GMP. These outsourcing efforts with respect to manufacturing preclinical and clinical supplies will result in a dependence on our suppliers to timely manufacture and deliver sufficient quantities of materials produced under GMP conditions to enable us to conduct planned preclinical studies, clinical trials and, if possible, to bring products to market in a timely manner.
Our current and anticipated future dependence upon these third-party manufacturers may adversely affect our ability to develop and commercialize product candidates on a timely and competitive basis. These manufacturers may not be able to produce material on a timely basis or manufacture material at the quality level or in the quantity required to meet our development timelines and applicable regulatory requirements. We may not be able to maintain or renew our existing third-party manufacturing arrangements, or enter into new arrangements, on acceptable terms, or at all. Our third-party manufacturers could terminate or decline to renew our manufacturing arrangements based on their own business priorities, at a time that is costly or inconvenient for us. If we are unable to contract for the production of materials in sufficient quantity and of sufficient quality on acceptable terms, our planned clinical trials may be delayed. Delays in preclinical or clinical testing could delay the filing of our IND applications and the initiation of clinical trials that we have currently planned.
16
Our third-party manufacturers may not be able to comply with the GMP regulations, other applicable FDA regulatory requirements or similar regulations applicable outside of the United States. Additionally, if we are required to enter into new supply arrangements, we may not be able to obtain approval from the FDA of any alternate supplier in a timely manner, or at all, which could delay or prevent the clinical development and commercialization of any related product candidates. Failure of our third-party manufacturers or us to obtain approval from the FDA or to comply with applicable regulations could result in sanctions being imposed on us, including fines, civil penalties, delays in or failure to grant marketing approval of our product candidates, injunctions, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of products and compounds, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect our business.
Because most of our expected future revenues are contingent upon collaborative and license agreements, we might not meet our strategic objectives.
Our ability to generate revenues in the near term depends on our ability to enter into additional collaborative agreements with third parties and to maintain the agreements we currently have in place. Our ability to enter into new collaborations and the revenue, if any, that may be recognized under these collaborations is highly uncertain. If we are unable to enter into new collaborations, our business prospects could be harmed, which could have an immediate adverse effect on the trading price of our stock.
To date, most of our revenues have been related to the research phase of each of our collaborative agreements. Such revenues are for specified periods, and the impact of such revenues on our results of operations is partially offset by corresponding research costs. Following the completion of the research phase of each collaborative agreement, additional revenue may come only from milestone payments and royalties, which may not be paid, if at all, until some time well into the future. The risk is heightened due to the fact that unsuccessful research efforts may preclude us from receiving any milestone payments under these agreements. Our receipt of revenue from collaborative arrangements is also significantly affected by the timing of efforts expended by us and our collaborators and the timing of lead compound identification. In late 2001, we recorded the first revenue from achievement of milestones in both the Pfizer and Johnson & Johnson collaborations. During 2002, we recorded our first milestone for both Novartis and Daiichi. Under many agreements, however, milestone payments may not be earned until the collaborator has advanced products into clinical testing, which may never occur or may not occur until some time well into the future. If we are not able to recognize revenue under our collaborations when and in accordance with our expectations or the expectations of industry analysts, this failure could harm our business and have an immediate adverse effect on the trading price of our stock.
Our business requires us to generate meaningful revenue from royalties and licensing agreements. To date, we have not received any revenue from royalties for the commercial sale of drugs, and we do not know when we will receive any such revenue, if at all. Likewise, we have not licensed any lead compounds or drug development candidates to third parties, and we do not know whether any such license will be entered into on acceptable terms in the future, if at all.
If our current corporate collaborations or license agreements are unsuccessful, our research and development efforts could be delayed.
Our strategy depends upon the formation and sustainability of multiple collaborative arrangements and license agreements with third parties in the future. We rely on these arrangements for not only financial resources, but also for expertise that we expect to need in the future relating to clinical trials, manufacturing, sales and marketing, and for licenses to technology rights. To date, we have entered into several such arrangements with corporate collaborators; however, we do not know if such third parties will dedicate sufficient resources or if any development or commercialization efforts by third parties will
17
be successful. Should a collaborative partner fail to develop or commercialize a compound or product to which it has rights from us, such failure might delay ongoing research and development efforts at Rigel because we might not receive any future milestone payments and we would not receive any royalties associated with such compound or product. In addition, the continuation of some of our partnered drug discovery and development programs may be dependent on the periodic renewal of our corporate collaborations. For example, the funded research phase of our collaboration with Pfizer has been completed and the development portion of our collaboration is ongoing at Pfizer.
Also, the research phase of our collaboration with Johnson & Johnson ended in December 2003. In addition, in May 2002, Novartis elected to conclude the research phases of our two initial joint projects in the autoimmunity and transplant rejection areas, after 42 months, effective November 2002 and February 2003, respectively. Generally, our current corporate collaboration agreements may terminate upon a breach or a change of control. We may not be able to renew these collaborations on acceptable terms, if at all, or negotiate additional corporate collaborations on acceptable terms, if at all.
Conflicts also might arise with collaborative partners concerning proprietary rights to particular compounds. While our existing collaborative agreements typically provide that we retain milestone payments and royalty rights with respect to drugs developed from certain derivative compounds, any such payments or royalty rights may be at reduced rates, and disputes may arise over the application of derivative payment provisions to such drugs, and we may not be successful in such disputes.
We are also a party to various license agreements that give us rights to use specified technologies in our research and development processes. The agreements pursuant to which we have in-licensed technology permit our licensors to terminate the agreements under certain circumstances. If we are not able to continue to license these and future technologies on commercially reasonable terms, our product development and research may be delayed.
If conflicts arise between our collaborators or advisors and us, any of them may act in their self-interest, which may be adverse to your interests.
If conflicts arise between us and our corporate collaborators or scientific advisors, the other party may act in its self-interest and not in the interest of our stockholders. Some of our corporate collaborators are conducting multiple product development efforts within each disease area that is the subject of the collaboration with us. In some of our collaborations, we have agreed not to conduct, independently or with any third party, any research that is competitive with the research conducted under our collaborations. Our collaborators, however, may develop, either alone or with others, products in related fields that are competitive with the products or potential products that are the subject of these collaborations. Competing products, either developed by our collaborators or to which our collaborators have rights, may result in their withdrawal of support for our product candidates.
If any of our corporate collaborators were to breach or terminate its agreement with us or otherwise fail to conduct the collaborative activities successfully and in a timely manner, the preclinical or clinical development or commercialization of the affected product candidates or research programs could be delayed or terminated. We generally do not control the amount and timing of resources that our corporate collaborators devote to our programs or potential products. We do not know whether current or future collaborative partners, if any, might pursue alternative technologies or develop alternative products either on their own or in collaboration with others, including our competitors, as a means for developing treatments for the diseases targeted by collaborative arrangements with us.
Our success is dependent on intellectual property rights held by us and third parties, and our interest in such rights is complex and uncertain.
Our success will depend to a large part on our own, our licensees' and our licensors' ability to obtain and defend patents for each party's respective technologies and the compounds and other
18
products, if any, resulting from the application of such technologies. We have approximately 135 pending patent applications and 39 issued patents in the United States that are owned or exclusively licensed in our field as well as pending corresponding foreign patent applications. In the future, our patent position might be highly uncertain and involve complex legal and factual questions. Additional uncertainty may result from because no consistent policy regarding the breadth of legal claims allowed in biotechnology patents has emerged to date. Accordingly, we cannot predict the breadth of claims allowed in our or other companies' patents.
Because the degree of future protection for our proprietary rights is uncertain, we cannot ensure that:
We rely on trade secrets to protect technology where we believe patent protection is not appropriate or obtainable. However, trade secrets are difficult to protect. While we require employees, collaborators and consultants to enter into confidentiality agreements, we may not be able to adequately protect our trade secrets or other proprietary information in the event of any unauthorized use or disclosure or the lawful development by others of such information.
We are a party to certain in-license agreements that are important to our business, and we generally do not control the prosecution of in-licensed technology. Accordingly, we are unable to exercise the same degree of control over this intellectual property as we exercise over our internally-developed technology. Moreover, some of our academic institution licensors, research collaborators and scientific advisors have rights to publish data and information in which we have rights. If we cannot maintain the confidentiality of our technology and other confidential information in connection with our collaborations, then our ability to receive patent protection or protect our proprietary information will be impaired. In addition, some of the technology we have licensed relies on patented inventions developed using U.S. government resources. The U.S. government retains certain rights, as defined by law, in such patents, and may choose to exercise such rights. Certain of our in-licenses may be terminated if we fail to meet specified obligations. If we fail to meet such obligations and any of our licensors exercise their termination rights, we could lose our rights under those agreements. If we lose any of our rights, it may affect the way we do business. In addition, because certain of our licenses are sublicenses, the actions of our licensors may affect our rights under those licenses.
If a dispute arises regarding the infringement or misappropriation of the proprietary rights of others, such dispute could be costly and result in delays in our research, development activities and partnering.
Our success will also depend, in part, on our ability to operate without infringing or misappropriating the proprietary rights of others. There are many issued patents and patent applications filed by third parties relating to products or processes that are similar or identical to ours
19
or our licensors, and others may be filed in the future. There can be no assurance that our activities, or those of our licensors, will not infringe patents owned by others. For example, in June 2002, we resolved a dispute with Inoxell A/S (formed as a spinout from Pharmexa—formally M&E Biotech) by entering into a global patent settlement concerning certain drug target identification technologies, which includes both cross-licensing and joint ownership to certain patents and allows for worldwide freedom of operation for both companies. We believe that there may be significant litigation in the industry regarding patent and other intellectual property rights, and we do not know if we or our collaborators would be successful in any such litigation. Any legal action against our collaborators or us claiming damages or seeking to enjoin commercial activities relating to the affected products, our methods or processes could:
If we are unable to obtain regulatory approval to market products in the United States and foreign jurisdictions, we might not be permitted to commercialize products from our research and development.
Due, in part, to the early stage of our product candidate research and development process, we cannot predict whether regulatory clearance will be obtained for any product that we, or our collaborative partners, hope to develop. Satisfaction of regulatory requirements typically takes many years, is dependent upon the type, complexity and novelty of the product and requires the expenditure of substantial resources. Of particular significance to us are the requirements relating to research and development and testing.
Before commencing clinical trials in humans in the United States, we, or our collaborative partners, will need to submit and receive approval from the FDA of an IND. Clinical trials are subject to oversight by institutional review boards and the FDA and:
While we have stated that we intend to file additional INDs, this is only a statement of intent, and we may not be able to do so because we may not be able to identify potential product candidates. In addition, the FDA may not approve any IND in a timely manner, or at all.
20
Before receiving FDA clearance to market a product, we must demonstrate that the product is safe and effective on the patient population that will be treated. Data obtained from preclinical and clinical activities are susceptible to varying interpretations that could delay, limit or prevent regulatory clearances. In addition, delays or rejections may be encountered based upon additional government regulation from future legislation or administrative action or changes in FDA policy during the period of product development, clinical trials and FDA regulatory review. Failure to comply with applicable FDA or other applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production or injunction, as well as other regulatory action against our potential products or us. Additionally, we have limited experience in conducting and managing the clinical trials necessary to obtain regulatory approval.
If regulatory clearance of a product is granted, this clearance will be limited to those disease states and conditions for which the product is demonstrated through clinical trials to be safe and efficacious. We cannot ensure that any compound developed by us, alone or with others, will prove to be safe and efficacious in clinical trials and will meet all of the applicable regulatory requirements needed to receive marketing clearance.
Outside the United States, our ability, or that of our collaborative partners, to market a product is contingent upon receiving a marketing authorization from the appropriate regulatory authorities. This foreign regulatory approval process typically includes all of the risks associated with FDA clearance described above and may also include additional risks.
If our competitors develop technologies that are more effective than ours, our commercial opportunity will be reduced or eliminated.
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. Many of the drugs that we are attempting to discover will be competing with existing therapies. In addition, a number of companies are pursuing the development of pharmaceuticals that target the same diseases and conditions that we are targeting. We face competition from pharmaceutical and biotechnology companies both in the United States and abroad.
Our competitors may utilize discovery technologies and techniques or partner with collaborators in order to develop products more rapidly or successfully than we, or our collaborators, are able to do. Many of our competitors, particularly large pharmaceutical companies, have substantially greater financial, technical and human resources than we do. In addition, academic institutions, government agencies and other public and private organizations conducting research may seek patent protection with respect to potentially competitive products or technologies and may establish exclusive collaborative or licensing relationships with our competitors.
We believe that our ability to compete is dependent, in part, upon our ability to create, maintain and license scientifically-advanced technology and upon our and our strategic partners' ability to develop and commercialize pharmaceutical products based on this technology, as well as our ability to attract and retain qualified personnel, obtain patent protection or otherwise develop proprietary technology or processes and secure sufficient capital resources for the expected substantial time period between technological conception and commercial sales of products based upon our technology. The failure by us or any of our collaborators in any of those areas may prevent the successful commercialization of our potential drug targets.
Our competitors might develop technologies and drugs that are more effective or less costly than any that are being developed by us or that would render our technology and potential drugs obsolete and noncompetitive. In addition, our competitors may succeed in obtaining the approval of the FDA or other regulatory agencies for product candidates more rapidly. Companies that complete clinical trials, obtain required regulatory agency approvals and commence commercial sale of their drugs before their competitors may achieve a significant competitive advantage, including certain patent and FDA
21
marketing exclusivity rights that would delay or prevent our ability to market certain products. Any drugs resulting from our research and development efforts, or from our joint efforts with our existing or future collaborative partners, might not be able to compete successfully with competitors' existing or future products or products under development or obtain regulatory approval in the United States or elsewhere.
Our ability to generate revenues will be diminished if our collaborative partners fail to obtain acceptable prices or an adequate level of reimbursement for products from third-party payors or government agencies.
The drugs we hope to develop may be rejected by the marketplace due to many factors, including cost. Our ability to commercially exploit a drug may be limited due to the continuing efforts of government and third-party payors to contain or reduce the costs of health care through various means. For example, in some foreign markets, pricing and profitability of prescription pharmaceuticals are subject to government control. In the United States, we expect that there will continue to be a number of federal and state proposals to implement similar government control. In addition, increasing emphasis on managed care in the United States will likely continue to put pressure on the pricing of pharmaceutical products. Cost control initiatives could decrease the price that any of our collaborators would receive for any products in the future. Further, cost control initiatives could adversely affect our collaborators' ability to commercialize our products and our ability to realize royalties from this commercialization.
Our ability to commercialize pharmaceutical products with collaborators may depend, in part, on the extent to which reimbursement for the products will be available from:
Significant uncertainty exists as to the reimbursement status of newly-approved healthcare products. Third-party payors, including Medicare, are challenging the prices charged for medical products and services. Government and other third-party payors increasingly are attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for new drugs and by refusing, in some cases, to provide coverage for uses of approved products for disease indications for which the FDA has not granted labeling approval. Third-party insurance coverage may not be available to patients for any products we discover and develop, alone or with collaborators. If government and other third-party payors do not provide adequate coverage and reimbursement levels for our products, the market acceptance of these products may be reduced.
If product liability lawsuits are successfully brought against us, we may incur substantial liabilities and may be required to limit commercialization of our products.
The testing and marketing of medical products entail an inherent risk of product liability. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our products although we are not currently aware of any specific causes for concern with respect to clinical liability claims. We currently do not have product liability insurance, and our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of pharmaceutical products we develop, alone or with corporate collaborators. We, or our corporate collaborators, might not be able to obtain insurance at a reasonable cost, if at all. While under various circumstances we are entitled to be indemnified against losses by our corporate collaborators, indemnification may not be available or adequate should any claim arise.
22
Our research and development efforts will be seriously jeopardized, if we are unable to attract and retain key employees and relationships.
As a small company with only 126 employees as of December 31, 2003, our success depends on the continued contributions of our principal management and scientific personnel and on our ability to develop and maintain important relationships with leading academic institutions, scientists and companies in the face of intense competition for such personnel. In particular, our research programs depend on our ability to attract and retain highly skilled chemists, other scientists, and regulatory and clinical personnel. If we lose the services of any of our personnel, our research and development efforts could be seriously and adversely affected. Our employees can terminate their employment with us at any time.
We depend on various scientific consultants and advisors for the success and continuation of our research and development efforts.
We work extensively with various scientific consultants and advisors. The potential success of our drug discovery and development programs depends, in part, on continued collaborations with certain of these consultants and advisors. We, and various members of our management and research staff, rely on certain of these consultants and advisors for expertise in our research, regulatory and clinical efforts. Our scientific advisors are not employees of ours and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. We do not know if we will be able to maintain such consulting agreements or that such scientific advisors will not enter into consulting arrangements, exclusive or otherwise, with competing pharmaceutical or biotechnology companies, any of which would have a detrimental impact on our research objectives and could have a material adverse effect on our business, financial condition and results of operations.
If we use biological and hazardous materials in a manner that causes injury or violates laws, we may be liable for damages.
Our research and development activities involve the controlled use of potentially harmful biological materials as well as hazardous materials, chemicals and various radioactive compounds. We cannot completely eliminate the risk of accidental contamination or injury from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for damages that result, and such liability could exceed our resources. We are also subject to federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. The cost of compliance with, or any potential violation of, these laws and regulations could be significant.
Our facilities are located near known earthquake fault zones, and the occurrence of an earthquake or other catastrophic disaster could cause damage to our facilities and equipment, which could require us to cease or curtail operations.
Our facilities are located in the San Francisco Bay Area near known earthquake fault zones and are vulnerable to significant damage from earthquakes. We are also vulnerable to damage from other types of disasters, including fires, floods, power loss, communications failures and similar events. If any disaster were to occur, our ability to operate our business at our facilities would be seriously, or potentially completely, impaired, and our research could be lost or destroyed. In addition, the unique nature of our research activities and of much of our equipment could make it difficult for us to recover from a disaster. The insurance we maintain may not be adequate to cover or losses resulting from disasters or other business interruptions.
23
If our officers, directors and largest stockholders choose to act together, they may be able to significantly affect our management and operations, acting in their best interests and not necessarily those of other stockholders.
Our directors, executive officers, principal stockholders and their affiliates beneficially owned approximately 57% of our common stock as of December 31, 2003. Accordingly, they collectively have the ability to significantly affect the election of all of our directors and the outcome of most corporate actions requiring stockholder approval. They may exercise this ability in a manner that advances their best interests and not necessarily those of other stockholders. In addition, the holders of approximately 8.4 million shares of common stock and warrants exercisable for approximately 1.6 million shares of our common stock are entitled to rights with respect to registration of those shares of common stock under the Securities Act.
On June 26, 2003, we completed a private placement with net proceeds of $45.0 million led by MPM Capital that included Frazier Healthcare, Alta Partners and HBM BioVentures. In the private placement, we issued 7,986,110 shares of our common stock at a price of $5.76 per share and warrants to purchase an additional 1,597,221 shares of our common stock at an exercise price of $5.76 per share. As a result of their combined approximate 64% ownership (without giving effect to the exercise of the warrants and based on 14,828,546 shares outstanding as of December 31, 2003), the investors obtained control over Rigel. The investors hold the requisite percentage of our outstanding shares so as to permit them, if they choose to act in concert, to take actions requiring stockholder approval without obtaining the approval of our other stockholders. For so long as MPM Capital holds at least 10% of the outstanding shares of our common stock, we are required to use our commercially reasonable best efforts to (i) cause two designees of MPM Capital to be nominated and elected to our board of directors; (ii) appoint one designee to serve on the nominating committee of our board of directors; and (iii) appoint one designee to serve on the compensation committee of our board of directors. These board appointments were completed in conjunction with the closing of the financing on June 26, 2003.
Our stock price may be volatile, and your investment in our stock could decline in value.
The market prices for our securities and those of other biotechnology companies have been highly volatile and may continue to be highly volatile in the future. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our common stock:
24
Anti-takeover provisions in our charter documents and under Delaware law may make an acquisition of us, which may be beneficial to our stockholders, more difficult.
Provisions of our amended and restated certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us, even if doing so would benefit our stockholders. These provisions:
In addition, Section 203 of the Delaware General Corporation Law, which imposes certain restrictions relating to transactions with major stockholders, may discourage, delay or prevent a third party from acquiring us.
Item 2. Properties
Our current facilities consist of approximately 147,000 square feet of research and office space located at 1180 Veterans Boulevard, South San Francisco, California. We believe our facilities are in good operating condition and that the real property leased is adequate for all present and near term uses.
Item 3. Legal Proceedings
None.
Item 4. Submission of Matters to a Vote of Security Holders
None.
25
Item 5. Market for the Registrant's Common Equity and Related Stockholder Matters
Our common stock has traded on the Nasdaq National Market under the symbol "RIGL" since November 29, 2000. The following table sets forth, for the periods indicated, the high and low sales prices for the common stock as reported by the Nasdaq National Market:
| |
High |
Low |
|||||
|---|---|---|---|---|---|---|---|
| Year Ended December 31, 2002 | |||||||
| First Quarter | $ | 45.90 | $ | 30.60 | |||
| Second Quarter | $ | 43.47 | $ | 19.80 | |||
| Third Quarter | $ | 26.73 | $ | 12.69 | |||
| Fourth Quarter | $ | 17.10 | $ | 9.45 | |||
| Year Ended December 31, 2003 | |||||||
| First Quarter | $ | 10.53 | $ | 5.22 | |||
| Second Quarter | $ | 13.50 | $ | 5.85 | |||
| Third Quarter | $ | 15.00 | $ | 7.18 | |||
| Fourth Quarter | $ | 19.20 | $ | 12.25 | |||
The sales prices in the above table reflect a one-for-nine reverse split of shares of our outstanding common stock effected on June 24, 2003. On February 27, 2004, the last reported sale price for our common stock on the Nasdaq National Market was $19.57 per share.
Holders
As of February 27, 2004, there were approximately 185 stockholders of record of our common stock.
Dividends
We have not paid dividends on our common stock and currently do not plan to pay any cash dividends in the foreseeable future.
Securities Authorized for Issuance Under Equity Compensation Plans
Information regarding securities authorized for issuance under equity compensation plans is incorporated by reference to the information set forth under the caption "Securities Authorized for Issuance Under Equity Compensation Plans" in our Proxy Statement for the Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission on or around April 30, 2004.
Sale of Unregistered Securities
On June 26, 2003, we completed a private placement with net proceeds of $45.0 million led by MPM Capital that included Frazier Healthcare, Alta Partners and HBM BioVentures. In the private placement, we issued 7,986,110 shares of our common stock at a price of $5.76 per share and warrants to purchase an additional 1,597,221 shares of our common stock at an exercise price of $5.76 per share. The securities were issued pursuant to an exemption from registration under Rule 506 under the Securities Act based on the fact that there were fewer than 35 purchasers of our securities in the private placement and our belief that each of the purchasers was an accredited investor. The securities sold in the private placement were subsequently registered for resale under a registration statement on Form S-3 filed with the Securities and Exchange Commission on July 10, 2003.
26
Item 6. Selected Financial Data
The following selected financial data should be read in conjunction with "Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Item 8. Financial Statements and Supplementary Data" included elsewhere in this annual report on Form 10-K.
| |
Fiscal Years Ended December 31, |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2003 |
2002 |
2001 |
2000 |
1999 |
||||||||||||
| |
(in thousands, except per share amounts) |
||||||||||||||||
| Statements of Operations Data: | |||||||||||||||||
| Contract revenues | $ | 11,055 | $ | 15,788 | $ | 15,303 | $ | 13,218 | $ | 8,984 | |||||||
| Costs and expenses: | |||||||||||||||||
| Research and development | 43,363 | 43,350 | 32,313 | 32,034 | 17,112 | ||||||||||||
| General and administrative | 8,519 | 9,454 | 7,950 | 6,689 | 3,952 | ||||||||||||
| 51,882 | 52,804 | 40,263 | 38,723 | 21,064 | |||||||||||||
| Loss from operations | (40,827 | ) | (37,016 | ) | (24,960 | ) | (25,505 | ) | (12,080 | ) | |||||||
| Loss on sale of property and equipment | (169 | ) | — | — | — | — | |||||||||||
| Interest income | 374 | 856 | 1,957 | 1,078 | 311 | ||||||||||||
| Interest expense | (575 | ) | (870 | ) | (802 | ) | (933 | ) | (597 | ) | |||||||
| Net loss | (41,197 | ) | (37,030 | ) | (23,805 | ) | (25,360 | ) | (12,366 | ) | |||||||
| Deemed dividend to Series E preferred stockholders | — | — | — | (10,133 | ) | — | |||||||||||
| Loss allocable to common stockholders | $ | (41,197 | ) | $ | (37,030 | ) | $ | (23,805 | ) | $ | (35,493 | ) | $ | (12,366 | ) | ||
| Loss per common share, basic and diluted | $ | (3.62 | ) | $ | (7.41 | ) | $ | (5.75 | ) | $ | (43.98 | ) | $ | (39.51 | ) | ||
| Weighted average common shares used in computing loss per common share, basic and diluted | 11,395 | 4,995 | 4,143 | 807 | 313 | ||||||||||||
| Pro forma loss per common share, basic and diluted | $ | (10.81 | ) | $ | (4.64 | ) | |||||||||||
| Shares used in computing pro forma loss per common share, basic and diluted | 3,283 | 2,666 | |||||||||||||||
| |
As of December 31, |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2003 |
2002 |
2001 |
2000 |
1999 |
|||||||||||
| |
(in thousands) |
|||||||||||||||
| Balance Sheet Data: | ||||||||||||||||
| Cash, cash equivalents and available-for-sale securities | $ | 46,500 | $ | 27,291 | $ | 33,415 | $ | 52,994 | $ | 5,836 | ||||||
| Working capital (deficiency) | 42,059 | 22,493 | 26,371 | 46,627 | (990 | ) | ||||||||||
| Total assets | 55,524 | 44,342 | 46,448 | 64,262 | 17,169 | |||||||||||
| Capital lease obligations, less current portion | 1,236 | 2,313 | 4,243 | 5,761 | 5,478 | |||||||||||
| Deferred stock compensation | (200 | ) | (772 | ) | (2,452 | ) | (5,792 | ) | (5,814 | ) | ||||||
| Accumulated deficit | (156,011 | ) | (114,814 | ) | (77,784 | ) | (53,979 | ) | (28,619 | ) | ||||||
| Total stockholders' equity | 39,973 | 25,441 | 28,941 | 49,010 | 756 | |||||||||||
The share numbers set forth in the table reflect a one-for-nine reverse split of shares of our outstanding common stock effected on June 24, 2003. See Notes to the Financial Statements for description of the number of shares used in the computation of basic and diluted and pro forma basic and diluted loss per common share.
27
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
Except for the historical information contained herein, the following discussion contains forward-looking statements that are based upon current expectations. Forward-looking statements involve risks and uncertainties and include statements related to:
When used herein, the words "believe," "anticipate," "expect," "estimate," "plan" and similar expressions are intended to identify such forward-looking statements. There can be no assurance that these statements will prove to be correct. Our actual results and the timing of events could differ significantly from those discussed here. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in "Risk Factors," as well as those discussed elsewhere in this annual report on Form 10-K. Rigel undertakes no obligation to update any of the forward-looking statements contained herein to reflect any future events or developments.
Overview
Rigel's mission is to become a source of novel, small-molecule drugs to meet large, unmet medical needs. We have three initial development programs: allergy/asthma, hepatitis C and rheumatoid arthritis. We have begun clinical testing of our first two product candidates, R112 for allergic rhinitis and R803 for hepatitis C, and plan to begin clinical trials of two additional product candidates, for the treatment of rheumatoid arthritis and asthma, by the end of 2004. We own the economic and commercial rights to these product candidates. Our business model is to develop a portfolio of product candidates and to take these through Phase II clinical trials, after which we intend to seek partners for completion of clinical trials, regulatory approval and marketing. We believe that our approach to drug discovery will enable us to commence clinical trials with one to two lead compounds each year.
Over the last year, we have matured into a drug development company with two product candidates in clinical trials and additional products expected to enter the clinical in 2004. Following is the status of our first four product candidates.
28
Corporate Collaborations
In addition to the preceding programs in which we retain all commercial and economic rights, we also carry on research and development programs in connection with our corporate collaborations. We currently have collaborations with four major pharmaceutical companies, including one with Janssen Pharmaceutica N.V., a division of Johnson & Johnson, relating to oncology therapeutics and diagnostics, one with Pfizer Inc. relating to asthma and allergy therapeutics, one with Novartis Pharma AG with four different programs relating to immunology, oncology and chronic bronchitis and one with Daiichi Pharmaceuticals Co., Ltd. in the area of oncology. These collaborations all have or had a research phase during which we receive or received funding based on the level of headcount allocated to a program. After the research phase concludes, we are entitled to certain milestones and royalties. Currently, only the Novartis oncology and chronic bronchitis programs and the Daiichi program are in the research phase of the agreements. In addition, we have a number of scientific collaborations with academic institutions and biotechnology companies under which we have in-licensed technology.
We are exploring new opportunities with existing and new potential collaborators. Our earliest partnerships focused on the early stages of drug discovery, specifically on target discovery and validation, while our collaboration with Johnson & Johnson has been expanded to also include both chemistry and compound HTS. Our recent collaboration with Daiichi focuses on drug discovery and development. We currently anticipate that in order to support our current research programs we will need to self-fund, at an increased rate of spending, our own research programs to later stages of development prior to partnering with collaborative partners. Therefore, it is expected that future collaborative partnerships may have an expanded focus and could include HTS, combinatorial and medicinal chemistry, preclinical evaluations and/or clinical development. For some programs, we may also seek to enter into collaborations for the development of compounds that we have discovered. For example, we have received preliminary human efficacy data for our lead compound R112, for the treatment of allergic rhinitis. We expect that this program could be the focus of our next corporate collaboration which we anticipate arranging in 2004.
Recent Developments
On June 24, 2003, we effected a one-for-nine reverse stock split of our outstanding common stock after our stockholders approved the proposal for a reverse split at our annual meeting of stockholders held on June 20, 2003. Immediately following the reverse split, we had a total of 5,159,519 shares of common stock outstanding.
On June 26, 2003, we completed a private placement with net proceeds of $45.0 million led by MPM Capital that included Frazier Healthcare, Alta Partners and HBM BioVentures. In the private placement, we issued 7,986,110 shares of our common stock at a price of $5.76 per share and warrants to purchase an additional 1,597,221 shares of our common stock at an exercise price of $5.76 per share. As a result of their then combined approximate 70.5% ownership (without giving effect to the exercise of the warrants and based on 13,167,556 shares outstanding as of June 30, 2003), the investors obtained control over Rigel.
On June 27, 2003, we initiated a rights offering pursuant to which non-transferable rights to purchase up to an aggregate of 1,736,111 shares of our common stock at a purchase price of $5.76 per share were offered to our stockholders of record as of April 29, 2003, other than certain stockholders affiliated with the investors in the private placement completed on June 26, 2003. Each such stockholder of record received one basic subscription right to purchase 0.4508 of a share of Rigel
29
common stock at $5.76 per share for each share owned as of the record date. By July 25, 2003, the expiration of the rights offering period, our stockholders had elected to purchase an aggregate of 1.6 million shares of our common stock for net proceeds to us of $9.1 million. The purchased shares were issued to the participating stockholders on July 31, 2003.
On June 27, 2003 we initiated an offer to exchange options, which was approved by our stockholders at our annual meeting on June 20, 2003, to purchase shares of our common stock with exercise prices equal to or greater than $9.00 per share currently outstanding under our 2000 Equity Incentive Plan, or 2000 Plan, 2001 Non-Officer Equity Incentive Plan and 2000 Non-Employee Directors' Stock Option Plan, for replacement options to purchase shares of our common stock to be granted under the 2000 Plan. There were outstanding eligible options to purchase an aggregate of 367,961 shares of our common stock as of June 26, 2003. Only officers, employees not on certain leaves of absence, consultants and non-employee members of Rigel's board of directors as of June 27, 2003, who continued to be employed through the offer expiration date of July 25, 2003, were eligible to participate in the offer. We offered to conduct the exchange with respect to eligible options on a one-for-one basis. On July 28, 2003, we accepted for cancellation options to purchase an aggregate of 344,207 shares of our common stock. On July 28, 2003, we granted replacement options to purchase an aggregate of 344,207 shares of our common stock at an exercise price of $9.20 per share, the fair market value on the date of grant. Subject to the continuation of the optionholders' employment, service as a consultant or service as a non-employee member of our board of directors, the replacement options will vest as follows: one-fifth of the shares covered by the replacement options will vest on the six-month anniversary of the date of grant; one-fifth of the shares covered by the replacement options will vest on the twelve-month anniversary of the date of grant; and three-fifths of the shares covered by the replacement options will vest in 24 equal monthly installments over the following two years. The replacement options will expire, at the latest, on the day three years and five business days after the date of grant (if they have not been forfeited earlier due to the optionholders' termination of employment, service as a consultant or service as a non-employee member of our board of directors). All replacement options, as well as the eligible options that were not surrendered under the original offer to exchange, are being treated for financial reporting purposes as variable awards. Therefore, we are recording a non-cash charge, generally for the intrinsic value of the options, reflecting increases and decreases (down to, but not below, the exercise price) in the price of our common stock, as compensation expense in connection with the replacement options and the eligible options that were not exchanged. We will continue to reflect increases and decreases in the price of our common stock in our statement of operations with respect to these options until they are exercised, forfeited or terminated. The higher the market value of our common stock, the greater the compensation expense. For the year ended December 31, 2003, we recorded a non-cash compensation charge of $1.1 million related to all options eligible for the replacement.
On February 25, 2004, we completed a follow-on offering in which we sold 2,850,000 shares and selling stockholders sold 315,000 shares of our common stock at a price of $20.00 per share. We received net proceeds of approximately $53,180,000 from the sale of shares offered by us, net of underwriting discounts and commissions and related expenses. We did not receive any proceeds from the sale of shares by the selling stockholders.
Critical Accounting Policies and the Use of Estimates
Our discussion and analysis of our financial condition and results of operations are based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates, including those related to terms of the research collaborations, investments, stock
30
compensation, impairment issues, the estimated useful life of assets and contingencies. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our financial statements.
We believe that there have been no significant changes in our critical accounting policies during the year ended December 31, 2003 as compared to those previously disclosed in our annual report on Form 10-K, as amended, for the year ended December 31, 2002.
Revenue Recognition
Non-refundable, up-front payments received in connection with research and development collaboration agreements, including technology access fees, are deferred and recognized on a straight-line basis over the relevant periods of continuing involvement, generally the research term.
Revenues related to collaborative research with our corporate collaborators are recognized as research services are performed over the related funding periods for each contract. Under these agreements, we are required to perform research and development activities as specified in each respective agreement. The payments received are not refundable and are generally based on a contractual cost per full-time equivalent employee working on the project. Research and development expenses under the collaborative research agreements approximate or exceed the revenue recognized under such agreements over the term of the respective agreements. Deferred revenue may result if we were not to incur the required level of effort during a specific period in comparison to funds received under the respective contracts.
Milestones are recognized pursuant to collaborative agreements upon the achievement of these specified at risk milestones.
Royalties will be recognized as earned in accordance with the contract terms when the third party results are reliably measurable and collectibility is reasonably assured.
Stock-based Compensation
We recorded no deferred stock compensation with respect to options granted to employees for the years ended December 31, 2003 and 2002. We recorded deferred stock compensation with respect to options granted to employees of approximately $0.3 million in the year ended December 31, 2001, representing the difference between the fair value of our common stock for financial reporting purposes on the date these options were granted and the exercise price. These amounts have been reflected as components of stockholders' equity, and the deferred expense is being amortized to operations over the vesting period of the options, generally four to five years, using the graded vesting method. As a result of our reduction in force on January 31, 2003, we recognized approximately $599,000 of stock-based compensation recovery associated with the unvested and cancelled options of the terminated employees that had previously been recognized under the graded vesting method of deferred compensation amortization. We amortized deferred stock compensation of $0.6 million, $1.0 million and $2.6 million for the years ended December 31, 2003, 2002 and 2001, respectively. At December 31, 2003, we had a total of $0.2 million remaining to be amortized over the remaining vesting periods of the stock options.
In addition to the amortization of the deferred stock compensation, we also record charges associated with the stock options eligible for repricing under the tender offer initiated on June 27, 2003. All replacement options, as well as the eligible options that were not surrendered under the
31
original offer to exchange, are being treated for financial reporting purposes as variable awards. Therefore, we are recording a non-cash charge, generally for the intrinsic value of the options reflecting increases and decreases (down to, but not below, the exercise price) in the price of our common stock as compensation expense in connection with the replacement options and the eligible options that were not exchanged. We will continue to reflect increases and decreases in the price of our common stock in our statement of operations with respect to these options until they are exercised, forfeited or terminated. The higher the market value of our common stock, the greater the compensation expense. For the year ended December 31, 2003, we recorded a non-cash compensation charge of $1.1 million related to all options eligible for the replacement.
We also record charges associated with options granted to consultants reflecting the periodic revaluation of outstanding unvested consultant options based upon the current market value of our common stock and other assumptions, including the expected future volatility of our stock price. We recognized stock-based compensation for revaluation of consultant options of $0.2 million for the year ended December 31, 2003. We recognized stock-based compensation recovery for revaluation of consultant options of $0.2 million and $0.5 million for the years ended December 31, 2002 and 2001, respectively. Even though the number of unvested outstanding options issued to consultants continues to decline, we expect to see continued fluctuations in the future as a portion of these options are revalued based on the changes in current market price of our common stock through the application of the graded vesting method.
Years Ended December 31, 2003, 2002 and 2001
| |
|
|
|
Aggregate Change |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Years Ended December 31, |
||||||||||||||
| |
2003 from 2002 |
2002 from 2001 |
|||||||||||||
| |
2003 |
2002 |
2001 |
||||||||||||
| |
(in thousands) |
||||||||||||||
| Contract revenues from collaborations | $ | 11,055 | $ | 15,788 | $ | 15,303 | $ | (4,733 | ) | $ | 485 | ||||
Revenues by collaborator were:
| |
|
|
|
Aggregate Change |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Years Ended December 31, |
|||||||||||||||
| |
2003 from 2002 |
2002 from 2001 |
||||||||||||||
| |
2003 |
2002 |
2001 |
|||||||||||||
| |
(in thousands) |
|||||||||||||||
| Daiichi | $ | 4,461 | $ | 971 | $ | — | $ | 3,490 | $ | 971 | ||||||
| Novartis | 4,119 | 11,074 | 8,576 | (6,955 | ) | 2,498 | ||||||||||
| Johnson & Johnson | 2,475 | 2,850 | 4,056 | (375 | ) | (1,206 | ) | |||||||||
| Pfizer | — | 893 | 2,671 | (893 | ) | (1,778 | ) | |||||||||
| Total | $ | 11,055 | $ | 15,788 | $ | 15,303 | $ | (4,733 | ) | $ | 485 | |||||
Revenues. Contract revenues from collaborations in 2003, 2002 and 2001 consisted primarily of research support and amortization of upfront fees from the continuation of our collaborations with Pfizer, Johnson & Johnson, Novartis and, in 2003 and 2002 only, Daiichi. Revenues in all years also included milestone payments from certain collaborators for targets delivered and accepted. The decrease in 2003 revenues of $4.7 million was primarily due to the termination of the research phase of the Novartis T-cell and B-cell programs offset by a $1.9 million milestone payment from Daiichi for the completion of a certain screening phase of the collaboration. Revenue was flat in 2002 as compared to 2001 primarily due to a combination of the end of the research phase of the Pfizer collaboration, offset by a full year of the angiogenesis program with Novartis and the commencement of the collaboration with Daiichi. Currently, only the Novartis oncology program and the Daiichi program are in the research phase of their agreements, and Novartis and Daiichi are obligated to pay us approximately
32
$2.7 million in research funding for 2004. We expect contract revenues from collaborations to be a significant component of our total revenues for the foreseeable future.
| |
|
|
|
Aggregate Change |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Years Ended December 31, |
||||||||||||||
| |
2003 from 2002 |
2002 from 2001 |
|||||||||||||
| |
2003 |
2002 |
2001 |
||||||||||||
| |
(in thousands) |
||||||||||||||
| Research and development expenses | $ | 43,363 | $ | 43,350 | $ | 32,313 | $ | 13 | $ | 11,037 | |||||
Research and Development. Even though research and development expenses were essentially flat from 2002 to 2003, there was a change in the mix of the respective costs. In 2003, there was a substantial increase in our facility costs associated with the move to our new building in February 2003 offset by reductions in contract chemistry, lab supplies, research headcount and costs associated with our intellectual property. Our clinical and preclinical costs increased as we continued to move our three development programs forward. The increase in 2002 of $11.1 million reflected primarily the continued expansion of our drug development infrastructure, the addition of both drug development and research headcount, increased outside contract efforts, increased preclinical activities, and the commencement of clinical trials. We expect our preclinical and clinical costs to increase substantially in 2004 as we plan to initiate a Phase II clinical trial for R112, a Phase I/II clinical trial for R803, a clinical trial for R406 and a clinical trial for a lead candidate from our asthma program.
The scope and magnitude of future research and development expenses are difficult to predict at this time given the number of studies that will need to be conducted for any of our potential products as well as our limited capital resources. In general, biopharmaceutical-development involves a series of steps—beginning with identification of a potential target and including, among others, proof of concept in animals and Phase I, II and III clinical studies in humans—each of which is typically more expensive than the previous step. Success in development therefore results in increasing expenditures. Our research and development expenditures currently include costs for scientific personnel, supplies, equipment, consultants, patent filings, sponsored research, allocated facility costs and costs related to clinical trials.
Because of the number of research projects we have ongoing at any one time, and the ability to utilize resources across several projects, the majority of our research and development costs are not directly tied to any individual project and are allocated among multiple projects. Our project management is based primarily on scientific data and supplemented by these cost allocations, which are based primarily on human resource time incurred on each project. As a result the costs allocated to a project do not necessarily reflect the actual costs of the project. Accordingly, we do not maintain actual cost incurred information for our projects on a project-by-project basis.
| |
|
|
|
Aggregate Change |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Years Ended December 31, |
||||||||||||||
| |
2003 from 2002 |
2002 from 2001 |
|||||||||||||
| |
2003 |
2002 |
2001 |
||||||||||||
| |
(in thousands) |
||||||||||||||
| General and administrative expenses | $ | 8,519 | $ | 9,454 | $ | 7,950 | $ | (935 | ) | $ | 1,504 | ||||
General and Administrative Expenses. The decrease in general and administrative expenses of $0.9 million in 2003 was primarily attributable to a reduction in employee costs as a result of cost-cutting measures that were initiated in September 2002 and January 2003 offset by higher facility costs. The increase in 2002 of $1.5 million was primarily attributable to higher employee costs and greater infrastructure costs to support the growing research and development activities. We expect that
33
general and administrative expenses will increase modestly in 2004 to support growing clinical development activities.
| |
Years Ended December 31, |
Aggregate Change |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2003 from 2002 |
2002 from 2001 |
||||||||||
| |
2003 |
2002 |
2001 |
|||||||||
| |
(in thousands) |
|||||||||||
| Loss on sale of property and equipment | $169 | $— | $— | $ | 169 | $ | — | |||||
Loss on Sale of Property and Equipment. In conjunction with our move to our new facilities in February 2003, we sold to the new tenant of our previous facility certain furniture and equipment that would no longer be needed at our new location. This sale resulted in cash proceeds of approximately $71,000 and a loss on sale of $169,000. The loss represents the remaining net book value of those assets less the cash received on the sale.
| |
|
|
|
Aggregate Change |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Years Ended December 31, |
|||||||||||||
| |
2003 from 2002 |
2002 from 2001 |
||||||||||||
| |
2003 |
2002 |
2001 |
|||||||||||
| |
(in thousands) |
|||||||||||||
| Net interest expense/(income) | $201 | $14 | $ | (1,155 | ) | $ | 187 | $ | (1,169 | ) | ||||
Net Interest Expense. Interest income results from our interest-bearing cash and investment balances, whereas interest expense is the result of our capital lease obligations associated with fixed asset purchases. In 2003 and 2002, interest expense exceeded interest income due primarily to a reduction in interest rates on our owned securities. In 2001, interest income exceeded interest expense due to higher interest rates earned on investment balances.
Effect of New Accounting Standards
In November of 2002, the Financial Accounting Standards Board issued Emerging Issues Task Force (referred to as EITF) Issue No. 00-21, "Revenue Arrangements with Multiple Deliverables." EITF Issue No. 00-21 addresses certain aspects of the accounting by a company for arrangements under which it will perform multiple revenue-generating activities. EITF Issue No. 00-21 addresses when and how an arrangement involving multiple deliverables should be divided into separate units of accounting. EITF Issue No. 00-21 provides guidance with respect to the effect of certain customer rights due to company nonperformance on the recognition of revenue allocated to delivered units of accounting. EITF Issue No. 00-21 also addresses the impact on the measurement and/or allocation of arrangement consideration of customer cancellation provisions and consideration that varies as a result of future actions of the customer or the company. Finally, EITF Issue No. 00-21 provides guidance with respect to the recognition of the cost of certain deliverables that are excluded from the revenue accounting for an arrangement. The provisions of EITF Issue No. 00-21 were applied to revenue arrangements entered into in fiscal periods beginning after June 15, 2003. Our adoption of the recognition requirements in July of 2003 of EITF Issue No. 00-21 did not have a material impact on our financial position or results of operations.
In January 2003, the Financial Accounting Standards Board, or FASB, issued Interpretation No. 46, or FIN 46, "Consolidation of Variable Interest Entities." FIN 46 requires a variable interest entity to be consolidated by a company if that company is subject to a majority of the risk of loss from the variable interest entity's activities or entitled to receive a majority of the entity's residual returns or both. A variable interest entity is a corporation, partnership, trust or any other legal structure used for business purposes that either (a) does not have equity investors with voting rights or (b) has equity investors that do not provide sufficient financial resources for the entity to support its activities. A variable interest entity often holds financial assets, including loans or receivables, real estate or other property. A variable interest entity may be essentially passive or it may engage in research and development or other activities on behalf of another company. However, the FASB deferred the
34
effective date for variable interest entities created before February 1, 2003 to the period ending March 31, 2004 for calendar year-end companies. Certain of the disclosure requirements apply to all financial statements issued after January 31, 2003, regardless of when the variable interest entity was established. Our adoption of the initial requirements in January of 2003 did not have an impact on our financial position and results of operations. The adoption of the remaining requirements of FIN 46 on January 1, 2004 is not expected to have a material impact on our financial position or results of operations.
In May 2003, the FASB issued Statements of Financial Accounting Standards No. 150, "Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity" (FAS 150). FAS 150 establishes standards for the classification and measurement of financial instruments with characteristics of both liabilities and equity. FAS 150 is effective for financial instruments entered into or modified after May 31, 2003 except for certain mandatorily redeemable financial instruments for which the FASB announced on November 5, 2003 deferred effective dates for certain provisions of FAS150. The adoption of FAS 150 and the subsequent deferred effective dates did not and are not expected to have a material effect on our financial position or results of operations.
Liquidity and Capital Resources
Cash Requirements
We have financed our operations from inception primarily through sales of equity securities, contract payments payable to us under our collaboration agreements and equipment financing arrangements. We believe that our existing capital resources, including the net proceeds to us from the recently completed offering in February 2004 of our common stock and anticipated proceeds from current and future collaborations, will be sufficient to support our current operating plan through the second quarter of 2006. Our operations will require significant additional funding in large part due to our research and development expenses, future preclinical and clinical-testing costs, the expansion of our facilities and the absence of any meaningful revenues for the foreseeable future. The amount of future funds needed will depend largely on the success of our collaborations and our research activities, and we do not know whether additional financing will be available when needed, or that, if available, we will obtain financing on terms favorable to our stockholders or us. We have consumed substantial amounts of capital to date, and operating expenditures are expected to increase over the next several years as we expand our research and development activities.
To the extent we raise additional capital by issuing equity securities, our stockholders would at that time experience substantial dilution. To the extent that we raise additional funds through collaboration and licensing arrangements, we may be required to relinquish some rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us. Our future funding requirements will depend upon many factors, including, but not limited to:
35
Insufficient funds may require us to delay, scale back or eliminate some or all of our research or development programs, to lose rights under existing licenses or to relinquish greater or all rights to product candidates at an earlier stage of development or on less favorable terms than we would otherwise choose or may adversely affect our ability to operate as a going concern.
As of December 31, 2003, we had $46.5 million in cash, cash equivalents and available-for-sale securities, as compared to $27.3 million as of December 31, 2002, an increase of $18.2 million. The increase was attributable to net proceeds of $45.0 million, after deducting offering costs, from the sale of 7,986,110 shares of our common stock and warrants to purchase 1,597,221 shares of our common stock to MPM Capital, Frazier Healthcare, Alta Partners and HBM BioVentures in a private placement completed in June 2003, as well as net proceeds of $9.1 million, after deducting offering costs, from the sale of 1,615,705 shares of our common stock to certain stockholders in a rights offering completed in July 2003. On February 25, 2004, we completed a follow-on offering in which we sold 2,850,000 shares of our common stock at a price of $20.00 per share, in which we received net proceeds of approximately $53,180,000, net of underwriting discounts and commissions and related expenses. We also invested $1.2 million in capital equipment and made debt service payments of $3.6 million in conjunction with our equipment financing arrangements. These payments were offset by $1.4 million of proceeds from a lease financing and $0.4 million from a cash advance from our landlord. For the three and nine months ended September 30, 2003 and 2002, we maintained an investment portfolio primarily in depository accounts and corporate commercial paper. Cash in excess of immediate requirements is invested with regard to liquidity and capital preservation. Wherever possible, we seek to minimize the potential effects of concentration and degrees of risk.
As of December 31, 2003, we had $3.5 million in capital lease obligations associated with our financed purchase of equipment and leasehold improvements. All existing equipment financing agreements as of December 31, 2003 are secured by the equipment financed, bear interest rates in a range of 7% to 15% and are due in monthly installments through 2006. On March 17, 2003, we amended an equipment lease agreement to allow for the buyout provision to be paid over a period of months rather than in a lump sum. The buyout provision of this amendment relates to approximately $2.9 million of original equipment and tenant improvement purchases. As a result of this amendment, we were committed to an additional $370,000 of payments through January 2004. As of December 31, 2003, we had approximately $39,000 remaining to be paid on this commitment. As of December 31, 2003, we had a total of $0.8 million available for draw down under all financing agreements.
During 2002, our office and research facility located at 240 East Grand in South San Francisco was leased under an operating lease that terminated in conjunction with a 15-year lease, signed in May 2001, for our current office and research facilities located at 1180 Veterans Blvd. in South San Francisco. Under the terms of the lease signed in 2001, we were to occupy our new facilities in late 2002 and were to concurrently terminate the lease of our former facility at 240 East Grand in South San Francisco. We determined that the 2001 lease for our current facility was an operating lease in accordance with FAS 13. In connection with the termination of the current 240 East Grand lease, we accelerated the amortization of tenant improvements and accrued rent charges over the expected
36
remaining life of the lease and incurred minimal costs in connection with the terminated lease. The 1180 Veterans Blvd. research and office facilities were constructed as a build-to-suit facility. Under the original lease for this new facility, we were obligated to fund approximately $18.0 million of the total tenant improvements. In October 2002, we amended this original lease to provide for a delay of the rent commencement date until February 1, 2003 and an increase in the tenant improvement allowance from the lessor to cover all of the expected remaining construction obligations on the facility. The lease was also amended to increase the future rental commitments to compensate for the delay of the rent commencement and the increase in the tenant improvement allowance. Since the amendment was considered a material change to the original lease, we reviewed the accounting treatment for this amended lease and again determined the lease to be an operating lease. We moved into the new facility during February 2003.
Prior to the signing of the amendment, we had been directly paying a portion of the pre-construction and construction costs related to tenant improvements in the new facility. These costs were being capitalized on our balance sheet as construction-in progress. Per the terms of the amendment, we estimated that the landlord would be responsible for reimbursing to us all of the costs that we had previously capitalized. Therefore, we reclassified these costs into a short-term asset "Receivable from Landlord" in our 2002 financial statements. The amount outstanding as of December 31, 2002 has been fully paid in 2003. We continue to incur minor costs associated with the new facility that we expect to recover from the landlord.
The following are our contractual commitments (by fiscal year) as of December 31, 2003 associated with debt obligations and lease obligations:
| |
Total |
2004 |
2005 - 2007 |
2008 - 2009 |
2010 - 2018 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(in thousands) |
|||||||||||||||
| Capital leases | $ | 3,807 | $ | 2,474 | $ | 1,333 | $ | — | $ | — | ||||||
| Facilities leases | 190,959 | 7,566 | 40,418 | 28,344 | 114,631 | |||||||||||
| Total | $ | 194,766 | $ | 10,040 | $ | 41,751 | $ | 28,344 | $ | 114,631 | ||||||
On January 31, 2003, we implemented a restructuring plan to reduce the rate of our cash consumption and better align our operating structure with current and expected future economic conditions. The restructuring plan included an immediate reduction in force of approximately 16 percent, or 25 employees, to 135 employees with reductions occurring in all functional areas. Two of our officers were included in this reduction in force. We also deferred a portion of certain officers' salaries.
As of December 31, 2003, we had federal net operating loss carryforwards of approximately $134.0 million to offset future taxable income. We also had federal research and development tax credit carryforwards of approximately $4.0 million. If not utilized, net operating loss and credit carryforwards will begin to expire in 2011. Utilization of the net operating losses and credits may be subject to a substantial annual limitation due to ownership change limitations provided by the Internal Revenue Code of 1986. The annual limitation may result in the expiration of our net operating losses and credits before they can be used. You should read Note 8 of the notes to our financial statements.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
The primary objective of our investment activities is to preserve principal while at the same time maximizing the income we receive from our investments without significantly increasing risk. Some of the securities in which we invest may have market risk. This means that a change in prevailing interest rates may cause the fair value amount of the investment to fluctuate. For example, if we hold a security that was issued with a fixed interest rate at the then-prevailing rate and the prevailing interest rate later rises, the market value amount of our investment will decline. To minimize this risk in the future, we
37
intend to maintain our portfolio of cash equivalents and short-term investments in a variety of securities, including commercial paper, money market funds and government and non-government debt securities. In 2003, 2002 and 2001, we maintained an investment portfolio primarily in depository accounts and corporate commercial paper. Due to the short-term nature of these investments, we believe we do not have a material exposure to interest rate risk arising from our investments. Therefore, no quantitative tabular disclosure is provided.
We have operated primarily in the United States, and all funding activities with our collaborators to date have been made in U.S. dollars. Accordingly, we have not had any exposure to foreign currency rate fluctuations.
38
Item 8. Financial Statements and Supplementary Data
INDEX TO FINANCIAL STATEMENTS
Rigel Pharmaceuticals, Inc.
| |
Page |
|
|---|---|---|
Report of Ernst & Young LLP, Independent Auditors |
40 |
|
Balance Sheets |
41 |
|
Statements of Operations |
42 |
|
Statement of Stockholders' Equity |
43 |
|
Statements of Cash Flows |
44 |
|
Notes to Financial Statements |
45 |
39
Report of Ernst & Young LLP, Independent Auditors
The Board of Directors and Stockholders
Rigel Pharmaceuticals, Inc.
We have audited the accompanying balance sheets of Rigel Pharmaceuticals, Inc. as of December 31, 2003 and 2002, and the related statements of operations, stockholders' equity, and cash flows for each of the three years in the period ended December 31, 2003. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Rigel Pharmaceuticals, Inc. at December 31, 2003 and 2002, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2003, in conformity with accounting principles generally accepted in the United States.
/S/ ERNST & YOUNG LLP
Palo
Alto, California
January 27, 2004, except for Note 9, as to
which the date is February 25, 2004.
40
RIGEL PHARMACEUTICALS, INC.
BALANCE SHEETS
(In thousands, except share and per share amounts)
| |
December 31, |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
2003 |
2002 |
||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 9,621 | $ | 26,535 | ||||
| Available-for-sale securities | 36,879 | 756 | ||||||
| Accounts receivable | 1,154 | 1,503 | ||||||
| Receivable from landlord | 133 | 6,175 | ||||||
| Prepaid expenses and other current assets | 2,326 | 1,894 | ||||||
| Total current assets | 50,113 | 36,863 | ||||||
| Property and equipment, net | 3,544 | 5,206 | ||||||
| Other assets | 1,867 | 2,273 | ||||||
| $ | 55,524 | $ | 44,342 | |||||
Liabilities and stockholders' equity |
||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 1,378 | $ | 3,460 | ||||
| Accrued compensation | 711 | 799 | ||||||
| Other accrued liabilities | 1,464 | 2,662 | ||||||
| Deferred revenue | 2,242 | 4,061 | ||||||
| Capital lease obligations | 2,259 | 3,388 | ||||||
| Total current liabilities | 8,054 | 14,370 | ||||||
| Long-term portion of capital lease obligations | 1,236 | 2,313 | ||||||
| Long-term portion of deferred revenue | 546 | 2,147 | ||||||
| Deferred rent | 5,297 | 71 | ||||||
| Other long-term liabilities | 418 | — | ||||||
| Commitments | ||||||||
| Stockholders' equity: | ||||||||
| Common stock, $0.001 par value; 100,000,000 shares authorized; 14,828,546 and 5,078,025 shares issued and outstanding in 2003 and 2002, respectively | 15 | 5 | ||||||
| Additional paid-in capital | 196,215 | 141,023 | ||||||
| Deferred stock compensation | (200 | ) | (772 | ) | ||||
| Accumulated other comprehensive loss | (13 | ) | (1 | ) | ||||
| Accumulated deficit | (156,011 | ) | (114,814 | ) | ||||
| 40,006 | 25,441 | |||||||
| Less treasury stock, at cost: 4,525 and no shares in 2003 and 2002, respectively | (33 | ) | — | |||||
| Total stockholders' equity | 39,973 | 25,441 | ||||||
| $ | 55,524 | $ | 44,342 | |||||
See accompanying notes.
41
RIGEL PHARMACEUTICALS, INC.
STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| |
Years ended December 31, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2003 |
2002 |
2001 |
||||||||
| Contract revenues from collaborations | $ | 11,055 | $ | 15,788 | $ | 15,303 | |||||
| Costs and expenses: | |||||||||||
| Research and development | 43,363 | 43,350 | 32,313 | ||||||||
| General and administrative | 8,519 | 9,454 | 7,950 | ||||||||
| 51,882 | 52,804 | 40,263 | |||||||||
| Loss from operations | (40,827 | ) | (37,016 | ) | (24,960 | ) | |||||
| Loss on sale of property and equipment | (169 | ) | — | — | |||||||
| Interest income | 374 | 856 | 1,957 | ||||||||
| Interest expense | (575 | ) | (870 | ) | (802 | ) | |||||
| Net loss | (41,197 | ) | (37,030 | ) | (23,805 | ) | |||||
| Net loss per common share, basic and diluted | $ | (3.62 | ) | $ | (7.41 | ) | $ | (5.75 | ) | ||
| Weighted average shares used in computing net loss per common share, basic and diluted | 11,395 | 4,995 | 4,143 | ||||||||
See accompanying notes.
42
RIGEL PHARMACEUTICALS, INC.
STATEMENT OF STOCKHOLDERS' EQUITY
(In thousands, except per share and per share amounts)
| |
Common Stock |
|
|
Accumulated Other Comprehensive Income (loss) |
|
|
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Additional Paid-in Capital |
Deferred Stock Compensation |
Accumulated Deficit |
Treasury Stock |
Total Stockholders' Equity |
||||||||||||||||||||
| |
Shares |
Amount |
|||||||||||||||||||||||
| Balance at December 31, 2000 | 4,089,354 | $ | 4 | $ | 108,775 | $ | (5,792 | ) | 2 | $ | (53,979 | ) | — | 49,010 | |||||||||||
| Net loss | — | — | — | — | — | (23,805 | ) | — | (23,805 | ) | |||||||||||||||
| Change in unrealized gain on available-for- sale securities | — | — | — | — | 42 | — | — | 42 | |||||||||||||||||
| Comprehensive loss | (23,763 | ) | |||||||||||||||||||||||
| Issuance of common stock upon exercise of options, warrants, and participation in Purchase Plan | 103,114 | — | 887 | — | — | — | — | 888 | |||||||||||||||||
| Issuance of warrant to purchase common stock for services | — | — | 683 | — | — | — | — | 683 | |||||||||||||||||
| Compensation recovery related to options granted to consultants | — | — | (510 | ) | — | — | — | — | (510 | ) | |||||||||||||||
| Deferred stock compensation | — | — | 285 | (285 | ) | — | — | — | — | ||||||||||||||||
| Amortization of deferred stock compensation, net of cancellations | — | — | (992 | ) | 3,625 | — | — | — | 2,633 | ||||||||||||||||
| Balance at December 31, 2001 | 4,192,468 | 4 | 109,128 | (2,452 | ) | 44 | (77,784 | ) | — | 28,941 | |||||||||||||||
| Net loss | — | — | — | — | — | (37,030 | ) | — | (37,030 | ) | |||||||||||||||
| Change in unrealized gain on available-for- sale securities | — | — | — | — | (45 | ) | — | — | (45 | ) | |||||||||||||||
| Comprehensive loss | (37,075 | ) | |||||||||||||||||||||||
| Issuance of common stock at $40.50 per share for cash, net of issuance costs | 777,778 | 1 | 29,421 | — | — | — | — | 29,428 | |||||||||||||||||
| Issuance of common stock at $38.70 per share for cash, net of issuance costs | 51,678 | — | 1,923 | — | — | — | — | 1,923 | |||||||||||||||||
| Issuance of common stock upon exercise of options and participation in Purchase Plan | 56,101 | — | 445 | — | — | — | — | 446 | |||||||||||||||||
| Issuance of warrants to purchase common stock for services | — | — | 1,018 | — | — | — | — | 1,018 | |||||||||||||||||
| Compensation recovery related to options granted to consultants | — | — | (196 | ) | — | — | — | — | (196 | ) | |||||||||||||||
| Amortization of deferred stock compensation, net of cancellations | — | — | (724 | ) | 1,680 | — | — | — | 956 | ||||||||||||||||
| Balance at December 31, 2002 | 5,078,024 | 5 | 141,023 | (772 | ) | (1 | ) | (114,814 | ) | — | 25,441 | ||||||||||||||
| Net loss | — | — | — | — | — | (41,197 | ) | — | (41,197 | ) | |||||||||||||||
| Change in unrealized gain on available-for- sale securities | — | — | — | — | (12 | ) | — | — | (12 | ) | |||||||||||||||
| Comprehensive loss | (41,209 | ) | |||||||||||||||||||||||
| Issuance of common stock at $5.76 per share for cash, net of issuance costs | 7,986,110 | 8 | 34,073 | — | — | — | — | 34,081 | |||||||||||||||||
| Issuance of common stock at $5.76 per share for cash, net of issuance costs | 1,615,705 | 2 | 9,103 | — | — | — | — | 9,105 | |||||||||||||||||
| Issuance of warrants to purchases common stock at $5.76 per share | — | — | 10,957 | — | — | — | — | 10,957 | |||||||||||||||||
| Fractional shares adjustment upon reverse split | (101 | ) | — | (1 | ) | — | — | — | — | (1 | ) | ||||||||||||||
| Issuance of common stock upon exercise of options and participation in Purchase Plan | 148,808 | — | 514 | — | — | — | — | 514 | |||||||||||||||||
| Purchase of common stock upon net exercise | — | — | — | — | — | — | (37 | ) | (37 | ) | |||||||||||||||
| Grant of treasury stock to an employee | — | — | 2 | — | — | — | 4 | 6 | |||||||||||||||||
| Compensation expense related to options granted to consultants | — | — | 159 | — | — | — | — | 159 | |||||||||||||||||
| Compensation expense related to repriced options | — | — | 1,125 | — | — | — | — | 1,125 | |||||||||||||||||
| Amortization of deferred stock compensation, net of cancellations | — | — | (740 | ) | 572 | — | — | — | (168 | ) | |||||||||||||||
| Balance at December 31, 2003 | 14,828,546 | $ | 15 | $ | 196,215 | $ | (200 | ) | $ | (13 | ) | $ | (156,011 | ) | $ | (33 | ) | $ | 39,973 | ||||||
See accompanying notes
43
RIGEL PHARMACEUTICALS, INC.
STATEMENTS OF CASH FLOWS
(In thousands)
| |
Years ended December 31, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2003 |
2002 |
2001 |
|||||||||
| Operating activities | ||||||||||||
| Net loss | $ | (41,197 | ) | $ | (37,030 | ) | $ | (23,805 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation and amortization | 2,664 | 4,868 | 4,127 | |||||||||
| Amortization of deferred stock compensation, net | 513 | 956 | 2,633 | |||||||||
| Noncash stock compensation (recovery) | 602 | (196 | ) | (510 | ) | |||||||
| Issuances of equity instruments for noncash benefits | 146 | 20 | — | |||||||||
| Loss on sale of fixed assets | 169 | — | — | |||||||||
| Changes in assets and liabilities: | ||||||||||||
| Accounts receivable | 349 | (350 | ) | (490 | ) | |||||||
| Prepaid expenses and other current assets, including receivable from landlord | 5,610 | (4,593 | ) | (939 | ) | |||||||
| Other assets | 266 | 201 | (551 | ) | ||||||||
| Accounts payable | (2,082 | ) | 1,480 | 638 | ||||||||
| Accrued compensation | (88 | ) | 128 | (53 | ) | |||||||
| Other accrued liabilities | (1,198 | ) | 74 | 408 | ||||||||
| Deferred revenue | (3,420 | ) | 704 | 2,734 | ||||||||
| Deferred rent and other long-term liabilities | 5,226 | (790 | ) | (173 | ) | |||||||
| Net cash used in operating activities | (32,440 | ) | (34,528 | ) | (15,981 | ) | ||||||
| Investing activities | ||||||||||||
| Purchases of available-for-sale securities | (42,135 | ) | (26,713 | ) | (47,511 | ) | ||||||
| Maturities of available-for-sale securities | 6,000 | 22,875 | 29,590 | |||||||||
| Sales of available-for-sale securities | — | 24,964 | — | |||||||||
| Proceeds from the sale of property and equipment | 71 | — | — | |||||||||
| Capital expenditures | (1,242 | ) | (1,635 | ) | (3,229 | ) | ||||||
| Net cash (used) provided in investing activities | (37,306 | ) | 19,491 | (21,150 | ) | |||||||
| Financing activities | ||||||||||||
| Proceeds from capital lease financing | 1,351 | 1,999 | 1,748 | |||||||||
| Payments on capital lease obligations | (3,557 | ) | (3,712 | ) | (3,047 | ) | ||||||
| Net proceeds from issuances of common stock and warrants | 54,620 | 31,797 | 888 | |||||||||
| Advance from landlord | 418 | — | — | |||||||||
| Net cash provided by (used in) financing activities | 52,832 | 30,084 | (411 | ) | ||||||||
| Net (decrease) increase in cash and cash equivalents | (16,914 | ) | 15,047 | (37,542 | ) | |||||||
| Cash and cash equivalents at beginning of period | 26,535 | 11,488 | 49,030 | |||||||||
| Cash and cash equivalents at end of period | $ | 9,621 | $ | 26,535 | $ | 11,488 | ||||||
| Supplemental disclosure of cash flow information | ||||||||||||
| Interest paid | $ | 576 | $ | 870 | $ | 802 | ||||||
| Schedule of non cash transactions | ||||||||||||
| Deferred stock compensation | $ | — | $ | — | $ | 285 | ||||||
| Issuance of warrants for services | $ | — | $ | 1,018 | $ | 683 | ||||||
See accompanying notes.
44
Rigel Pharmaceuticals, Inc.
NOTES TO FINANCIAL STATEMENTS
In this annual report on Form 10-K, "Rigel," "we," "us" and "our" refer to Rigel Pharmaceuticals, Inc. "common stock" refers to Rigel's common stock, par value $0.001 per share.
1. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Nature of operations and basis of presentation
We were incorporated in the state of Delaware on June 14, 1996. We are engaged in the discovery and development of a broad range of new small molecule product candidates. On June 24, 2003, we effected a one-for-nine reverse stock split of our outstanding common stock, after our stockholders approved the proposal for a reverse split at our annual meeting of stockholders held on June 20, 2003. As a result of the reverse stock split, each outstanding share of common stock automatically converted into one-ninth of a share of common stock, with the par value of each share of common stock remaining at one tenth of one cent ($.001) per share. Accordingly, common stock share and per share amounts for all periods presented have been adjusted to reflect the impact of the reverse stock split.
Use of estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from these estimates.
Stock Award Plans
We have elected to continue to follow Accounting Principles Board Opinion No. 25, or APB 25, "Accounting for Stock Issued to Employees," to account for employee stock options because the alternative fair value method of accounting prescribed by Statement of Financial Accounting Standards, or FAS, No. 123, as amended by FAS No. 148 "Accounting for Stock-Based Compensation," requires the use of option valuation models that were not developed for use in valuing employee stock options. Under APB 25, the intrinsic value method of accounting, no compensation expense is recognized because the exercise price of our employee stock options equals the market price of the underlying stock on the date of grant.
Pro forma information regarding net loss and net loss per share has been determined as if we had accounted for our employee stock options and employee stock purchase plan under the fair value method prescribed by FAS 123, as amended by FAS 148. The fair value for these options was estimated at the date of grant using the Black-Scholes model with the following weighted-average assumptions for the years ended December 31, 2003, 2002 and 2001: risk-free interest rates of 1.3%, 2.1% and 3.7%, respectively; volatility of 1.00, 0.85 and 0.65 respectively; an expected option life of five years; and no dividend yield.
45
For purposes of pro forma disclosures, the estimated fair value of the options is amortized to expense over the vesting period of the options. Our pro forma information follows (in thousands, except per share amounts):
| |
Years Ended December 31, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2003 |
2002 |
2001 |
||||||||
| Net loss applicable to common stockholders—as reported: | $ | (41,197 | ) | $ | (37,030 | ) | $ | (23,805 | ) | ||
| Less: Total stock-based compensation determined under APB 25 | 957 | 955 | 2,633 | ||||||||
| Add: Total stock-based compensation expense determined under the fair value based method for all awards | 3,066 | 3,391 | 2,808 | ||||||||
| Pro forma net loss | $ | (43,306 | ) | $ | (39,466 | ) | $ | (23,980 | ) | ||
| Basic and diluted net loss per common share: | |||||||||||
| As reported | $ | (3.62 | ) | $ | (7.41 | ) | $ | (5.75 | ) | ||
| Pro forma | (3.80 | ) | (7.90 | ) | (5.79 | ) | |||||
Cash, cash equivalents and available-for-sale securities
We consider all highly liquid investments in debt securities with a remaining maturity from the date of purchase of 90 days or less to be cash equivalents. Cash equivalents consist of money market funds and corporate debt securities. Our short-term investments include obligations of governmental agencies and corporate debt securities. By policy, we limit the concentration of credit risk by diversifying our investments among a variety of high credit-quality issuers.
All cash equivalents and short-term investments are classified as available-for-sale. Available-for-sale securities are carried at fair value which approximates amortized cost at December 31, 2003 and 2002. Unrealized gains (losses) are reported in stockholders' equity and included in other comprehensive income (loss). Fair value is estimated based on available market information. The cost of securities sold is based on the specific identification method. For the years ended December 31, 2003, 2002 and 2001, gross realized gains and losses on available-for-sale securities were not material. See Note 4 for a summary of available-for-sale securities at December 31, 2003 and 2002.
Fair value of financial instruments
Financial instruments, including cash and cash equivalents, accounts receivable, accounts payable, accrued liabilities and accrued compensation are carried at cost or amortized cost, which management believes approximates fair value.
Derivative financial instruments and hedging activities
All derivatives are required to be recognized on the balance sheet at fair value. Derivatives that are not designated as hedges must be adjusted to fair value through earnings. If the derivative is designated and qualifies as a hedge, depending on the nature of the hedge, changes in the fair value of the derivative are either offset against the change in fair value of assets, liabilities, or firm commitments through earnings or recognized in other comprehensive income until the hedged item is recognized in earnings. The ineffective portion of a derivative's change in fair value will be immediately
46
recognized in earnings. We do not hold derivative financial instruments and do no currently engage in hedging activities.
Property and equipment
Property and equipment are stated at cost. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets, which range from three to seven years. Leasehold improvements are amortized using the straight-line method over the estimated useful lives of the assets or the term of the lease, whichever is shorter.
Revenue recognition
Non-refundable, up-front payments received in connection with research and development collaboration agreements, including technology access fees, are deferred and recognized on a straight-line basis over the relevant periods of continuing involvement, generally the research term.
Revenues related to collaborative research with our corporate collaborators are recognized as research services are performed over the related development funding periods for each contract. Under these agreements, we are required to perform research and development activities as specified in each respective agreement. The payments received are not refundable and are generally based on a contractual cost per full-time equivalent employee working on the project. Research and development expenses under the collaborative research agreements approximate or exceed the revenue recognized under such agreements over the term of the respective agreements. Deferred revenue may result if we were not to incur the required level of effort during a specific period in comparison to funds received under the respective contracts.
Milestones are recognized pursuant to collaborative agreements upon the achievement of these specified at risk milestones.
Royalties will be recognized as earned in accordance with the contract terms when the third party results are reliably measurable and collectibility is reasonably assured.
Research and development
Research and development expenses include costs for scientific personnel, supplies, equipment, consultants, research sponsored by us, allocated facility costs, costs related to clinical trials, and stock-based compensation expense. All such costs are charged to research and development expense as incurred. Collaboration agreements generally specify minimum levels of research effort required to be performed by us.
Impairment of long-lived assets
We adopted FAS 144, "Accounting for the Impairment or Disposal of Long-Lived Assets," on January 1, 2002. Our adoption of FAS 144 did not have a material impact on our financial position or results of operations. Long-lived assets and certain identifiable intangible assets to be held and used are reviewed for impairment when events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable.
47
Segment reporting
We have determined that we operate in only one segment.
Contingencies
We are subject to claims related to the patent protection of certain of our technologies. We are required to assess the likelihood of any adverse judgments or outcomes to these matters as well as potential ranges of probable losses. A determination of the amount of reserves required, if any, for these contingencies is made after careful analysis of each individual issue. The required reserves may change in the future due to new developments in each matter or changes in approach such as a change in settlement strategy in dealing with these matters.
Net loss per share
Net loss per share has been computed according to Financial Accounting Standards Board Statement No. 128, "Earnings Per Share," which requires disclosure of basic and diluted earnings per share. Basic earnings per share excludes any dilutive effects of options, shares subject to repurchase, warrants and convertible securities. Diluted earnings per share includes the impact of potentially dilutive securities.
During all periods presented, we had securities outstanding which could potentially dilute basic earnings per share in the future, but were excluded from the computation of diluted net loss per share, as their effect would have been antidilutive. These outstanding securities consist of the following (in thousands, except per share information):
| |
December 31, |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
2003 |
2002 |
2001 |
||||||
| Outstanding options | 2,081 | 718 | 640 | ||||||
| Warrants | 1,725 | 128 | 33 | ||||||
| Weighted average exercise price of options | $ | 8.31 | $ | 31.23 | $ | 31.32 | |||
| Weighted average exercise price of warrants | $ | 7.17 | $ | 24.84 | $ | 45.27 | |||
Recent accounting pronouncements
In November of 2002, the Financial Accounting Standards Board issued Emerging Issues Task Force (referred to as EITF) Issue No. 00-21, "Revenue Arrangements with Multiple Deliverables." EITF Issue No. 00-21 addresses certain aspects of the accounting by a company for arrangements under which it will perform multiple revenue-generating activities. EITF Issue No. 00-21 addresses when and how an arrangement involving multiple deliverables should be divided into separate units of accounting. EITF Issue No. 00-21 provides guidance with respect to the effect of certain customer rights due to company nonperformance on the recognition of revenue allocated to delivered units of accounting. EITF Issue No. 00-21 also addresses the impact on the measurement and/or allocation of arrangement consideration of customer cancellation provisions and consideration that varies as a result of future actions of the customer or the company. Finally, EITF Issue No. 00-21 provides guidance with respect to the recognition of the cost of certain deliverables that are excluded from the revenue accounting for an arrangement. The provisions of EITF Issue No. 00-21 were applied to revenue
48
arrangements entered into in fiscal periods beginning after June 15, 2003. Our adoption of the recognition requirements in July of 2003 of EITF Issue No. 00-21 did not have a material impact on our financial position or results of operations.
In January 2003, the Financial Accounting Standards Board, or FASB, issued Interpretation No. 46, or FIN 46, "Consolidation of Variable Interest Entities." FIN 46 requires a variable interest entity to be consolidated by a company if that company is subject to a majority of the risk of loss from the variable interest entity's activities or entitled to receive a majority of the entity's residual returns or both. A variable interest entity is a corporation, partnership, trust or any other legal structure used for business purposes that either (a) does not have equity investors with voting rights or (b) has equity investors that do not provide sufficient financial resources for the entity to support its activities. A variable interest entity often holds financial assets, including loans or receivables, real estate or other property. A variable interest entity may be essentially passive or it may engage in research and development or other activities on behalf of another company. However, the FASB deferred the effective date for variable interest entities created before February 1, 2003 to the period ending March 31, 2004 for calendar year-end companies. Certain of the disclosure requirements apply to all financial statements issued after January 31, 2003, regardless of when the variable interest entity was established. Our adoption of the initial requirements in January of 2003 did not have an impact on our financial position and results of operations. The adoption of the remaining requirements of FIN 46 on January 1, 2004 is not expected to have a material impact on our financial position or results of operations.
In May 2003, the FASB issued Statements of Financial Accounting Standards No. 150, "Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity" (FAS 150). FAS 150 establishes standards for the classification and measurement of financial instruments with characteristics of both liabilities and equity. FAS 150 is effective for financial instruments entered into or modified after May 31, 2003 except for certain mandatorily redeemable financial instruments for which the FASB announced on November 5, 2003 deferred effective dates for certain provisions of FAS150. The adoption of FAS 150 and the subsequent deferred effective dates did not and are not expected to have a material effect on our financial position or results of operations.
2. SPONSORED RESEARCH AND LICENSE AGREEMENTS
Research agreements
In December 1998, we entered into a research collaboration agreement with Johnson and Johnson Pharmaceutical and Development, LLC to research and identify novel targets for drug discovery. Under the terms of the contract, Johnson & Johnson paid a one-time non-refundable, non-creditable fee and provided support for research activities during the research period which concluded in December 2003, as well as various milestones. Johnson & Johnson is obligated to pay us various milestones and royalties if certain conditions are met.
In January 1999, we entered into a two-year collaborative research agreement with Pfizer Inc. to discover and develop various molecular targets. Upon signing of the agreement, Pfizer was obligated to pay a one-time, nonrefundable, noncreditable fee. Under the terms of the contract, Pfizer provided support for research for two years and is obligated to pay us various milestones and royalties if certain conditions are met. On January 25, 2001, Pfizer notified us that it was electing to exercise its option to extend the funded research portion of the collaboration one additional year to January 31, 2002 and
49
then extended it again for one additional month to February 28, 2002. In February 2002, the research phase of our collaboration with Pfizer concluded with Pfizer accepting a total of seven validated targets.
In May 1999, we entered into a broad collaboration with Novartis Pharma AG, whereby we and Novartis agreed to work on up to five different research programs to identify various targets for drug development. Two programs were initiated in 1999 while the third program to be conducted at Novartis was initiated on January 1, 2000. In July 2001, we expanded our collaboration with Novartis with the initiation of our angiogenesis program, the fourth and final program in our Novartis collaboration. Pursuant to the expanded Novartis collaboration, we received a $4.0 million up-front payment from Novartis, which will be recognized as revenue ratably through July 2004. In addition, the expanded collaboration provides that the angiogenesis research program will be carried out at Rigel, provides for research reimbursement through July 2004 and includes potential future milestones and royalty payments to Rigel. Novartis notified us that it has chosen not to exercise its option for a second program of research that would have been carried out at Novartis. In May 2002, Novartis elected to conclude the research phases of our two initial joint projects in the autoimmunity and transplant rejection areas, after 42 months each, effective in November 2002 and February 2003, respectively. Pursuant to the collaboration agreement, Novartis had the option to end the research phase on these programs after either 24 months or 42 months. For all programs, Novartis is obligated to provide payment for various milestones and royalties if certain conditions in the collaboration agreement are met
In August 2002, we signed an agreement for the establishment of a collaboration with Daiichi to pursue research related to a specific target from a novel class of drug targets called ligases that control cancer cell proliferation through protein degradation. Per the agreement, the research phase of this collaboration is for three years. We are working with Daiichi to discover and develop cancer pharmaceutical drugs. Under the terms of the collaboration agreement, Daiichi has paid us $0.9 million upfront, two milestone payments totaling $3.7 million, is obligated to pay us ongoing research support and may become obligated to pay us certain other milestones payments. In addition, we are entitled to receive royalties on any commercialized products to emerge from the collaboration.
The initial stages of the collaboration focused on the development of the assay for the specific target and the initiation of HTS to identify therapeutic molecules we and Daiichi would like to advance to later stages of drug development. Under terms of the agreement, we retain the rights to co-develop and co-promote products resulting from this collaboration in North America while Daiichi retains co-development and promotion rights in the remainder of the world.
3. SIGNIFICANT CONCENTRATIONS
For the year ended December 31, 2003, Daiichi, Novartis and Johnson and Johnson accounted for 41%, 37% and 22% of total revenues, respectively. For the year ended December 31, 2002, Novartis, Johnson and Johnson, Daiichi and Pfizer accounted for 70%, 18%, 6% and 6% of total revenues, respectively. For the year ended December 31, 2001, Pfizer, Johnson and Johnson and Novartis accounted for 17%, 27% and 56% of total revenues, respectively. Accounts receivable relate mainly to these collaborative partners. Rigel does not require collateral or other security for accounts receivable.
50
4. AVAILABLE-FOR-SALE SECURITIES
Available-for-sale securities consist of the following (in thousands):
| |
Amortized Cost and Fair Value at December 31, |
|||||
|---|---|---|---|---|---|---|
| |
2003 |
2002 |
||||
| Money market funds | $ | 9,621 | $ | 26,535 | ||
| Corporate commercial paper | 36,879 | 756 | ||||
| $ | 46,500 | $ | 27,291 | |||
| Reported as: | ||||||
| Cash and cash equivalents | $ | 9,621 | $ | 26,535 | ||
| Available-for-sale securities | 36,879 | 756 | ||||
| $ | 46,500 | $ | 27,291 | |||
At December 31, 2003, the available-for-sale securities had maturities of less than one year, with an average maturity of approximately 122 days.
There were no material gross realized gains or losses from sales of securities in the periods presented. Unrealized gains and losses on available-for-sale securities were not material at December 31, 2003 or 2002.
5. PROPERTY AND EQUIPMENT
Property and equipment consists of the following (in thousands):
| |
Years Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2003 |
2002 |
|||||
| Laboratory and office equipment | $ | 17,448 | $ | 16,691 | |||
| Leasehold improvements | — | 3,175 | |||||
| Construction in progress | — | 197 | |||||
| Total property and equipment | 17,448 | 20,063 | |||||
| Less accumulated depreciation and amortization | (13,904 | ) | (14,857 | ) | |||
| Property and equipment, net | $ | 3,544 | $ | 5,206 | |||
At December 31, 2003 and 2002, equipment under capital leases was approximately 8.7 million and $15.0 million, respectively, with accumulated depreciation and amortization of approximately $13.9 million and $13.3 million, respectively. Amortization expense was $24,000, $1,710,000 and $1,042,000 for the years ended December 31, 2003, 2002 and 2001, respectively.
51
6. LONG-TERM OBLIGATIONS
At December 31, 2003, future minimum lease payments and obligations under all noncancelable leases were as follows (in thousands):
| |
Capital Leases |
Operating Leases |
||||
|---|---|---|---|---|---|---|
| 2004 | $ | 2,474 | $ | 7,566 | ||
| 2005 | 1,031 | 13,872 | ||||
| 2006 | 302 | 13,034 | ||||
| 2007 | — | 13,512 | ||||
| 2008 | — | 13,945 | ||||
| 2009 and thereafter | — | 129,030 | ||||
| Total minimum payments required | 3,807 | $ | 190,959 | |||
| Less amount representing interest | (312 | ) | ||||
| Present value of future lease payments | 3,495 | |||||
| Less current portion | (2,259 | ) | ||||
| Noncurrent obligations under capital leases | 1,236 | |||||
During 2002, our office and research facility located at 240 East Grand in South San Francisco was leased under an operating lease terminated in conjunction with a 15-year lease for our current office and research facilities at 1180 Veterans Blvd. in South San Francisco signed in May 2001. Under the terms of the lease signed in 2001, we were to occupy our new facility in late 2002 and were to concurrently terminate our lease of our former facility at 240 East Grand in South San Francisco. We determined that the 2001 lease was an operating lease in accordance with FAS 13. In connection with the termination of the current 240 East Grand lease, we accelerated the amortization of tenant improvements and accrued rent charges over the expected remaining life of the lease and incurred minimal costs in connection with the terminated lease. The 1180 Veterans Blvd. research and office facilities were constructed as a build-to-suit facility. Under the original lease for this new facility we were obligated to fund approximately $18.0 million of the total tenant improvements. In October 2002, we amended this original lease to provide for a delay of the rent commencement date until February 1, 2003 and an increase in the tenant improvement allowance from the lessor to cover the remaining construction obligations on the facility. The lease was also amended to increase the future rental commitments to compensate for the delay of the rent commencement and the increase in the tenant improvement allowance. Since the amendment was considered a material change to the original lease, we revisited the proper accounting treatment for this lease per FAS 13 and again determined the lease to be an operating lease. We moved into the new facilities during February 2003.
Prior to the signing of the amendment, we had been directly paying a portion of the pre-construction and construction costs related to the tenant improvements in the new facility. These costs were being capitalized on our balance sheet as construction-in progress. We estimated that the landlord would be responsible for all of the costs that we had previously capitalized. Therefore, we reclassified these costs into a short-term asset "Receivable from Landlord" in our 2002 financial statements. The amount outstanding as of December 31, 2002 was fully paid in 2003. We continue to incur minor costs associated with the new facility that we expect to recover from the landlord.
52
Rent expense under all operating leases amounted to approximately $13,515,000, $1,897,000 and $2,167,000 for the years ended December 31, 2003, 2002 and 2001, respectively.
In June 1998, we entered into a equipment lease line agreement for up to $3,000,000, which was fully utilized in June 1999. The lease period was for four years. The interest on each lease is fixed at the time of the draw down with the interest rates ranging from 6.5% to 7.2%. In March 2003, we amended this agreement to allow for the buyout provision to be paid out over a period of months rather than in a lump sum. The buyout provision of this amendment relates to approximately $2.9 million of original equipment and tenant improvement purchases. As a result of this amendment, we were committed to an additional $370,000 of payments through January 2004. As of December 31, 2003, we had approximately $40,000 remaining to be paid on this commitment.
In June 1999 and August 1999, we entered into two additional equipment lease line agreements for an aggregate total of $6,000,000, or $3,000,000 each additional lease agreement. These lines were fully utilized in May 2000. The lease period was for four years. The interest on each lease is fixed at the time of the draw down with the interest rates ranging from 11.7% to 15.0%. Both lines have buyout provisions of approximately 10% and 12.5% of the original utilized line amount for the lines entered in June 1999 and August 1999, respectively.
In August 2000, we entered into an additional equipment lease line agreement for an aggregate total of $5,000,000. We utilized $4,148,000 of the facility but have no remaining availability under the facility. The lease period was for four years. The interest on the lease is fixed at the time of the draw down with the interest rates ranging from 10.6% to 14.6%. This line has a bargain purchase buyout provision of $1.
In January 2002, we entered into an additional equipment lease line agreement for an aggregate total of $2,000,000. This line was fully utilized in August 2002. The lease period was for 37 months. The interest on the lease is fixed at the time of the draw down with the interest rates ranging from 11.5% to 11.7%. This line has a buyout provision of approximately 10% of the original utilized line amount.
In December 2002, we entered into an additional equipment lease line agreement for an aggregate total of $2,000,000. We originally had the ability to draw down on this line until December 2003, but this date was extended to March 2004 through an agreement reached in 2003. As of December 31, 2003, $1.4 million of this line had been utilized. The lease period will be for three years. The interest on the lease is fixed at the time of any draw down. This line has a buyout provision of either the fair-market value of the equipment or 10% of the original utilized line amount.
Obligations under all leases are secured by the assets financed under the leases.
53
7. STOCKHOLDERS' EQUITY
Common stock
In January 2002, we issued 777,778 shares of common stock in a registered direct offering to certain institutional investors at a price of $40.50 per share under our shelf registration statement. We received net proceeds of approximately $29.4 million after deducting commissions and offering costs. In February 2002, we issued 51,678 shares of common stock in a registered direct offering to a certain institutional investor at a price of $38.70 per share under our shelf registration statement. We received net proceeds of approximately $1.8 million after deducting commissions and offering costs.
In June 2003, we completed a private placement with net proceeds of $45.0 million led by MPM Capital that included Frazier Healthcare, Alta Partners and HBM BioVentures. In the private placement, we issued 7,986,110 shares of our common stock at a price of $5.76 per share plus warrants (described below).
In June 2003, we initiated a rights offering pursuant to which non-transferable rights to purchase up to an aggregate of 1,736,111 shares of our common stock at a purchase price of $5.76 per share were offered to our stockholders of record as of April 29, 2003, other than certain stockholders affiliated with the investors in the private placement completed on June 26, 2003. Each such stockholder of record received one basic subscription right to purchase 0.4508 of a share of Rigel common stock at $5.76 per share for each share owned as of the record date. By July 25, 2003, the expiration of the rights offering period, our stockholders had elected to purchase an aggregate of 1,616,705 shares of our common stock for net proceeds to us of $9.1 million. The shares were issued to the participating stockholders on July 31, 2003.
Warrants
In conjunction with the equipment lease line executed in April 1997, we issued a warrant to purchase 19,444 shares of series B preferred stock at an exercise price of $7.20 per share. Upon the closing of our initial public offering, this warrant automatically converted to a warrant to purchase 19,444 shares of common stock at $7.20 per share. This warrant was exercised in June 2001 and was no longer outstanding.
In conjunction with the equipment lease line executed in June 1998, we issued a warrant to purchase 14,620 shares of series C preferred stock at an exercise price of $10.26 per share. Upon the closing of our initial public offering, this warrant automatically converted to a warrant to purchase 14,620 shares of common stock at $10.26 per share. This warrant was exercised in June 2001 and is no longer outstanding.
In conjunction with the facilities lease entered into in June 1998, we issued three warrants to purchase an aggregate of 16,666 shares of common stock at an exercise price of $10.26 per share. The warrants are exercisable at any time up to November 28, 2007, the seventh anniversary of the closing of our initial public offering.
In conjunction with the facilities lease entered into in May 2001, we issued a warrant to purchase 16,666 shares of our common stock at an exercise price of $80.21 per share, a 15% premium to market at the time of issuance. This warrant will expire on May 16, 2006. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $683,000. This amount has been capitalized in other long term assets and is being amortized into expense over the life of the lease.
54
In conjunction with the equipment lease line executed in January 2002, we issued a warrant to purchase 2,645 shares of our common stock at an exercise price of $37.80 per share. This warrant will expire on January 31, 2007. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $66,000. This amount has been capitalized in other long-term assets and is being amortized into expense over the payment period of the equipment lease line.
In conjunction with the equipment lease line executed in July 2002, we issued a warrant to purchase 15,432 shares of our common stock at an exercise price of $24.30 per share. This warrant will expire on July 12, 2012. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $251,000. This amount was completely expensed in 2002 in conjunction with the termination of the line.
In conjunction with the amendment of our master lease agreement for our 1180 Veterans Blvd. facility entered into in October 2002, we issued a warrant to purchase 55,555 shares of our common stock at an exercise price of $17.73 per share. This warrant will expire on October 18, 2007. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $565,000. This amount has been capitalized in other long term assets and is being amortized into expense over the life of the lease.
In conjunction with the equipment lease line executed in December 2002, we issued a warrant to purchase 20,768 shares of our common stock at an exercise price of $9.63 per share. This warrant will expire on December 23, 2007. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $136,000. This amount has been capitalized in other long-term assets and is being amortized into expense over the payment period of the equipment lease line.
In conjunction with the financing completed in June 2003, we issued warrants to purchase an additional 1,597,221 shares of our common stock at an exercise price of $5.76 per share. These warrants will expire on June 26, 2008. The fair value of these warrants, as determined by the Black-Scholes valuation model, was approximately $11.0 million. This amount has been allocated within "Additional paid-in capital" in our financial statements.
Stock option plans
In January 2000, we adopted the 2000 Equity Incentive Plan, or 2000 Plan, which was approved in March 2000 by our stockholders. The 2000 Plan is an amendment and restatement of the 1997 Stock Option Plan. Under the 2000 Plan, incentive stock options, nonstatutory stock options and shares of common stock may be granted to our employees, directors and consultants. In July 2001, we adopted the 2001 Non-Officer Equity Incentive Plan, or 2001 Plan. Under the 2001 Plan, which was not approved by our stockholders, nonstatutory stock options may be granted to our employees and consultants. In April 2003, our board of directors approved an amendment to the 2000 Plan, which was subsequently approved by our stockholders at our annual meeting in June 2003, to (i) merge the 2001 Plan into the 2000 Plan and to terminate the 2001 Plan, (ii) increase the number of shares authorized for issuance under the 2000 Plan (including the available reserve from the merging of the 2001 Plan) by 1,600,000 shares of common stock (iii) add an evergreen feature that provides for automatic annual increases in the total number of shares reserved for issuance under the 2000 Plan. Options originally granted under our 2000 Plan and 2001 Plan expire no later than ten years from the date of grant. The option price of each incentive stock option shall be at least 100% of the fair value on the date of grant,
55
and the option price for each nonstatutory stock option shall be not less than 85% of the fair value on the date of grant, as determined by the board of directors. Options may be granted with different vesting terms from time to time, not to exceed five years from the date of grant. As of December 31, 2003, a total of 807,666 shares of common stock have been authorized for issuance under the 2000 Plan.
In August 2000, we adopted the 2000 Non-Employee Directors Stock Option Plan, or Directors' Plan, which was approved in September 2000 by our stockholders. Under the original plan, each non-employee director who becomes a director of Rigel would be automatically granted a nonstatutory stock option to purchase 2,222 shares of common stock on the date on which such person first becomes a director. At each board meeting immediately following each annual meeting of stockholders, beginning with the board meeting following the 2001 Annual Stockholders Meeting, each non-employee director would automatically be granted a nonstatutory option to purchase 556 shares of common stock. In April 2003, our board of directors approved an amendment to the Directors' Plan, which was subsequently approved by our stockholders at our annual meeting in June 2003, to (i) increase the number of shares authorized for issuance under the Directors' Plan by 66,667 shares of common stock, (ii) increase the size of the initial grants to 6,667 shares of common stock, (iii) increase the size of the annual grant to 1,667 shares of common stock. The exercise price of options under the Directors' Plan will be equal to the fair market value of the common stock on the date of grant. The maximum term of the options granted under the Directors' Plan is ten years. All grants under the Directors' Plan will vest monthly over two years from date of grant. The Directors' Plan will terminate in September 2009, unless terminated earlier in accordance with the provisions of the Directors' Plan. As of December 31, 2003, a total of 86,513 shares of common stock have been authorized for issuance under the Directors' Plan.
In June 2003, we initiated an offer to exchange options to purchase shares of our common stock with exercise prices equal to or greater than $9.00 per share currently outstanding under the 2000 Plan, the 2001 Plan and the Directors' Plan, for replacement options to purchase shares of our common stock to be granted under the 2000 Plan. There were outstanding eligible options to purchase an aggregate of 367,961 shares of our common stock as of June 26, 2003. Only officers, employees not on certain leaves of absence, consultants and non-employee members of Rigel's board of directors as of June 27, 2003, who continued to be employed through the offer expiration date of July 25, 2003, were eligible to participate in the offer. We offered to conduct the exchange with respect to eligible options on a one-for-one basis. On July 28, 2003, we accepted for cancellation options to purchase an aggregate of 344,207 shares of our common stock. On July 28, 2003, we granted replacement options to purchase an aggregate of 344,207 shares of our common stock at an exercise price of $9.20 per share, the fair market value on the date of the grant. Subject to the continuation of the optionholders' employment, service as a consultant or service as a non-employee member of our board of directors, the replacement options will vest as follows: one-fifth of the shares covered by the replacement options will vest on the six-month anniversary of the date of grant; one-fifth of the shares covered by the replacement options will vest on the twelve-month anniversary of the date of grant; and three-fifths of the shares covered by the replacement options will vest in 24 equal monthly installments over the following two years. The replacement options will expire, at the latest, on the day three years and five business days after the date of grant (if they have not been forfeited earlier due to the optionholders' termination of employment, service as a consultant or service as a non-employee member of our board of directors). All replacement options, as well as the eligible options that were not surrendered under the original
56
offer to exchange, are being treated for financial reporting purposes as variable awards. Therefore, we are recording a non-cash charge, generally for the intrinsic value of the options, reflecting increases and decreases (down to, but not below, the exercise price) in the price of our common stock in compensation expense in connection with the replacement options and the eligible options that were not exchanged. We will continue to reflect increases and decreases in the price of our common stock in our statement of operations with respect to these options until they are exercised, forfeited or terminated. The higher the market value of our common stock, the greater the compensation expense. For the year ended December 31, 2003, we recorded a non-cash compensation charge of $1.1 million related to all options eligible for the replacement.
Activity under all of the option plans through December 31, 2003 was as follows:
| |
Shares Available For Grant |
Number of Options |
Weighted-Average Exercise Price |
|||||
|---|---|---|---|---|---|---|---|---|
| Outstanding at December 31, 2000 | 203,672 | 629,977 | $ | 24.30 | ||||
| Authorized for grant | 388,889 | — | ||||||
| Granted | (114,656 | ) | 114,656 | $ | 55.89 | |||
| Exercised | — | (61,377 | ) | $ | 5.31 | |||
| Cancelled | 43,137 | (43,137 | ) | $ | 27.45 | |||
| Outstanding at December 31, 2001 | 521,042 | 640,119 | $ | 31.32 | ||||
| Granted | (184,768 | ) | 184,768 | $ | 30.15 | |||
| Exercised | — | (36,760 | ) | $ | 2.34 | |||
| Cancelled | 69,783 | (69,783 | ) | $ | 44.31 | |||
| Options outstanding at December 31, 2002 | 406,057 | 718,344 | $ | 31.23 | ||||
| Authorized for grant | 1,962,689 | — | ||||||
| Granted | (1,991,162 | ) | 1,991,162 | $ | 8.56 | |||
| Exercised | — | (111,692 | ) | $ | 2.02 | |||
| Cancelled | 516,595 | (516,595 | ) | $ | 42.53 | |||
| Options outstanding at December 31, 2003 | 894,179 | 2,081,219 | $ | 8.31 | ||||
| Exercisable at December 31, 2003 | 293,164 | $ | 6.77 | |||||
| Exercisable at December 31, 2002 | 380,292 | $ | 29.88 | |||||
| Exercisable at December 31, 2001 | 247,065 | $ | 24.57 | |||||
| Weighted average fair value of options granted during 2003 | $ | 7.21 | ||||||
| Weighted average fair value of options granted during 2002 | $ | 20.43 | ||||||
| Weighted average fair value of options granted during 2001 | $ | 32.13 | ||||||
57
Details of Rigel's stock options by exercise price is as follows:
| |
Options Outstanding |
|
Options Exercisable |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise Price |
Number of Outstanding Options |
Weighted-Average Remaining Contractual Life |
Weighted-Average Exercise Price |
Number of Options |
Weighted-Average Exercise Price |
|||||||
| $0.90 - $6.57 | 136,852 | 5.26 | $ | 1.99 | 122,987 | $ | 1.89 | |||||
| $8.15 - $9.20 | 1,883,491 | 8.31 | $ | 8.40 | 160,231 | $ | 8.23 | |||||
| $14.20 - $14.75 | 50,591 | 9.73 | $ | 14.75 | 359 | $ | 14.51 | |||||
| $37.80 - $41.58 | 9,024 | 6.54 | $ | 40.45 | 8,337 | $ | 40.37 | |||||
| $73.53 - $82.13 | 1,261 | 7.47 | $ | 74.45 | 1,250 | $ | 74.39 | |||||
| $0.90 - $82.13 | 2,081,219 | 8.14 | $ | 8.31 | 293,164 | $ | 6.77 | |||||
We granted 100 shares of common stock options to consultants for services in 2003. We also cancelled and regranted 16,636 common stock options to consultants in association with the repricing on July 28, 2003. We granted 7,222 and 12,778 common stock options to consultants in exchange for services in 2002 and 2001. We recognized stock-based compensation for revaluation of consultant options of $0.2 million for the year ended December 31, 2003. We recognized stock-based compensation recovery for revaluation of consultant options of $0.2 million and $0.5 million for the years ended December 31, 2002 and 2001, respectively.
We recorded no deferred stock compensation with respect to options granted to employees for the years ended December 31, 2003 and 2002. We recorded deferred stock compensation with respect to options granted to employees of approximately $0.3 million in the years ended December 31, 2001, representing the difference between the deemed fair value of our common stock for financial reporting purposes on the date these options were granted and the exercise price. These amounts have been reflected as components of stockholders' equity, and the deferred expense is being amortized to operations over the vesting period of the options, generally four to five years, using the graded vesting method. As a result of our reduction in force on January 31, 2003, we recognized approximately $599,000 of stock-based compensation recovery associated with the unvested and cancelled options of the terminated employees which had previously been recognized under the graded vesting method of deferred compensation amortization. We amortized deferred stock compensation of $0.6 million, $1.0 million and $2.6 million for the years ended December 31, 2003, 2002 and 2001, respectively. At December 31, 2003, we had a total of $0.2 million remaining to be amortized over the remaining vesting periods of the stock options.
2000 employee stock purchase plan
In August 2000, we adopted the 2000 Employee Stock Purchase Plan, or Purchase Plan, which was approved in September 2000 by our stockholders. In April 2003, our board of directors approved an amendment to the Purchase Plan, which was subsequently approved by our stockholders at our annual meeting in June 2003, to (i) increase the number of shares authorized for issuance under the Purchase Plan by 66,667 shares of common stock, (ii) change the evergreen feature of the plan. The amendment provides that the increase in the number of shares reserved automatically pursuant to the evergreen feature will be equal to the least of 1% of the outstanding shares on the date of the annual increase, 88,889 shares or such amount as may be determined by the board. The Purchase Plan permits eligible employees to purchase common stock at a discount through payroll deductions during defined offering
58
periods. The price at which the stock is purchased is equal to the lower of 85% of the fair market value of the common stock on the first day of the offering or 85% of the fair market value of our common stock on the purchase date. The initial offering period commenced on the effective date of our initial public offering. We issued 37,112, 19,273 and 13,300 shares of common stock during 2003, 2002 and 2001, respectively, pursuant to the Purchase Plan at an average price of $8.45 per share, $18.63 per share, and $44.82 per share in 2003, 2002 and 2001, respectively. For 2003, 2002 and 2001, the weighted average fair value of stock issued under the Purchase Plan was $4.33, $1.68 and $2.42, respectively. A total of 44,444 shares of Rigel's common stock were initially reserved for issuance under the Purchase Plan. The Purchase Plan provides for annual increases in the number of shares available for issuance under the Purchase Plan on each anniversary date of the effective date of the offering. The number of shares reserved for future issuance under the Purchase Plan was increased by 66,667, 44,444 and 41,843 during 2003, 2002 and 2001, respectively.
Reserved shares
As of December 31, 2003, we had reserved shares of common stock for future issuance as follows:
| |
December 31, 2003 |
||
|---|---|---|---|
| Warrants | 1,724,953 | ||
| Incentive stock plans. | 2,975,398 | ||
| Purchase Plan | 127,713 | ||
| Total | 4,828,064 | ||
8. INCOME TAXES
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred tax assets are as follows:
| |
December 31, |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
2003 |
2002 |
||||||
| Deferred tax assets | ||||||||
| Net operating loss carryforwards | $ | 46,440 | $ | 31,300 | ||||
| Research and development credits | 6,425 | 5,500 | ||||||
| Capitalized research and development expenses | 7,643 | 3,500 | ||||||
| Other, net | 2,417 | 4,100 | ||||||
| Total deferred tax assets | 62,925 | 44,400 | ||||||
| Valuation allowance | (62,925 | ) | (44,400 | ) | ||||
| Net deferred tax assets | $ | — | $ | — | ||||
Realization of deferred tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance. The valuation allowance increased by $18.5 million, $18.2 million and $5.2 million during 2003, 2002 and 2001, respectively.
59
Included in the valuation allowance balance at December 31, 2003 is $1.6 million related to the exercise of stock options which are not reflected as an expense for financial reporting purposes. Accordingly, any future reduction in the valuation allowance relating to this amount will be credited directly to equity and not reflected as an income tax benefit in the statement of operations.
As of December 31, 2003, we had net operating loss carryforwards for federal income tax purposes of approximately $134.0 million, which expire beginning in the year 2011 and federal research and development tax credits of approximately $4.0 million, which will begin to expire in 2012.
Utilization of the net operating loss and credit may be subject to a substantial annual limitation due to the "change in ownership" provisions of the Internal Revenue Code of 1986 (IRC) and similar state provisions. The annual limitation may result in the expiration of net operating losses and credits before utilization.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets.
9. SUBSEQUENT EVENTS
Follow-on Public Offering
On February 25, 2004, we completed a follow-on offering in which we sold 2,850,000 shares and selling stockholders sold 315,000 shares of our common stock at a price of $20.00 per share. We received net proceeds of approximately $53,180,000 from the sale of shares offered by us, net of underwriting discounts and commissions and related expenses. We did not receive any proceeds from the sale of shares by the selling stockholders.
10. SELECTED QUARTERLY FINANCIAL DATA (unaudited, in thousands, except per share amounts)
| |
Year Ended December 31, 2003 |
Year Ended December 31, 2002 |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Q1 |
Q2 |
Q3 |
Q4 |
Q1 |
Q2 |
Q3 |
Q4 |
|||||||||||||||||
| Revenue | $ | 4,497 | $ | 2,349 | $ | 2,103 | $ | 2,106 | $ | 4,098 | $ | 4,337 | $ | 3,653 | $ | 3,700 | |||||||||
| Net loss | $ | (7,800 | ) | $ | (10,466 | ) | $ | (11,069 | ) | $ | (11,862 | ) | $ | (8,372 | ) | $ | (10,446 | ) | $ | (10,142 | ) | $ | (8,070 | ) | |
| Net loss per common share, basic and diluted | $ | (1.53 | ) | $ | (1.90 | ) | $ | (0.78 | ) | $ | (0.80 | ) | $ | (1.74 | ) | $ | (2.07 | ) | $ | (2.01 | ) | $ | (1.59 | ) | |
| Weighted average shares used in computing net loss per common share, basic and diluted | 5,089 | 5,496 | 14,224 | 14,796 | 4,812 | 5,038 | 5,057 | 5,067 | |||||||||||||||||
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures. Based on our management's evaluation (with the participation of our chief executive officer and chief financial officer), our chief executive officer and chief financial officer have concluded that, subject to limitations described below, our disclosure
60
controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended), were effective as of December 31, 2003 to ensure that information required to be disclosed by us in this annual report on Form 10-K was recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms.
Changes in Internal Controls. There were no changes in our internal controls over financial reporting during the quarter ended December 31, 2003 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on the Effectiveness of Controls. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the controls are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within a company have been detected. Accordingly, our disclosure controls and procedures are designed to provide reasonable, not absolute, assurance that the objectives of our disclosure control system are met and, as set forth above, our chief executive officer and chief financial officer have concluded, based on their evaluation as of the end of the period covered by this annual report on Form 10-K, that our disclosure controls and procedures were sufficiently effective to provide reasonable assurance that the objectives of our disclosure control system were met.
61
Item 10. Directors and Executive Officers of the Registrant
Information regarding directors and executive officers is incorporated by reference to the information set forth under the caption "Directors and Executive Officers" in our Proxy Statement for the Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission on or around April 30, 2004.
In 2003, we adopted a code of ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. Our code of ethics is on our website at http://www.rigel.com/pdf/codeofconduct.pdf in connection with "Investor Resources" materials. If we make any substantive amendments to the code or grant any waiver from a provision of the code applicable to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on a Form 8-K.
Item 11. Executive Compensation
Information regarding executive compensation is incorporated by reference to the information set forth under the caption "Executive Compensation" in our Proxy Statement for the Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission on or around April 30, 2004.
Item 12. Security Ownership of Certain Beneficial Owners and Management
Information regarding security ownership of certain beneficial owners and management is incorporated by reference to the information set forth under the caption "Security Ownership of Certain Beneficial Owners and Management" and "Equity Compensation Plan Information" in our Proxy Statement for the Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission on or around April 30, 2004.
Item 13. Certain Relationships and Related Transactions
Information regarding certain relationships and related transactions is incorporated by reference to the information set forth under the caption "Certain Transactions" in our Proxy Statement for the Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission on or around April 30, 2004.
Item 14. Principal Accounting Fees and Services.
Information regarding principal accounting fees and services is incorporated by reference to the information set forth under the caption "Ratification of Independent Auditors" in our Proxy Statement for the Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission on or around April 30, 2004.
Item 15. Exhibits, Financial Statement Schedules and Reports on Form 8-K
62
| 3.1(1) | Amended and Restated Certificate of Incorporation. | |
| 3.2(1) | Amended and Restated Bylaws. | |
| 4.1(1) | Specimen Common Stock Certificate. | |
| 4.2(1) | Amended and Restated Investor Rights Agreement between Rigel and holders of Rigel's Series B, Series C, Series D and Series E preferred stock, dated February 3, 2000. | |
| 4.3(1) | Form of warrant to purchase shares of common stock. | |
| 4.7(11) | Amended and Restated Warrant issued to Kwacker Limited for the purchase of shares of common stock. | |
| 4.8(7) | Warrant issued to TBCC Funding Trust II for the purchase of shares of common stock. | |
| 4.9(8) | Warrant issued to Comerica Bank-California for the purchase of shares of common stock | |
| 4.10(11) | Warrant issued to Kwacker Limited for the purchase of shares of common stock. | |
| 4.11(11) | Warrant issued to Lighthouse Capital Partners IV, L.P. to purchase shares of common stock. | |
| 4.12(12) | Warrant issued to Alta BioPharma Partners II, L.P. to purchase shares of common stock. | |
| 4.13(12) | Warrant issued to Alta California Partners, L.P. to purchase shares of common stock. | |
| 4.14(12) | Warrant issued to Alta Embarcadero BioPharma Partners II, LLC to purchase shares of common stock. | |
| 4.15(12) | Warrant issued to Alta Embarcadero Partners, LLC to purchase shares of common stock. | |
| 4.16(12) | Warrant issued to HBM BioVentures (Cayman) Ltd. to purchase shares of common stock. | |
| 4.17(12) | Warrant issued to MPM BioVentures III, L.P. to purchase shares of common stock. | |
| 4.18(12) | Warrant issued to MPM BioVentures III-QP, L.P. to purchase shares of common stock. | |
| 4.19(12) | Warrant issued to MPM BioVentures III GmbH & Co. Beteiligungs KG to purchase shares of common stock. | |
| 4.20(12) | Warrant issued to MPM BioVentures III Parallel Fund, L.P. to purchase shares of common stock. | |
| 4.21(12) | Warrant issued to MPM Asset Management Investors 2003 BVIII LLC to purchase shares of common stock. | |
| 4.22(12) | Warrant issued to MPM BioEquities Master Fund, L.P. to purchase shares of common stock. | |
| 4.23(12) | Second Investor Rights Agreement between Rigel and certain investors, dated June 26, 2003. | |
| 10.1(1) | Form of Indemnity Agreement. | |
| 10.2(11)(2) | 2000 Equity Incentive Plan, as amended. | |
| 10.3(1)(2) | Form of Stock Option Agreement pursuant to 2000 Equity Incentive Plan. | |
| 10.4(1)(2) | 2000 Employee Stock Purchase Plan. | |
| 10.5(1)(2) | 2000 Non-Employee Directors' Stock Option Plan. | |
| 10.6(1) | Collaboration Agreement between Rigel and Janssen Pharmaceutica N.V., dated December 4, 1998. | |
| 10.7(1) | Collaborative Research and License Agreement between Rigel and Pfizer Inc., dated January 31, 1999. | |
| 10.8(1) | Collaboration Agreement between Rigel and Novartis Pharma AG, dated May 26, 1999. | |
| 10.9(1)(3) | License and Research Agreement between Rigel and Cell Genesys, Inc., dated September 2, 1999. | |
| 10.10(1) | Collaborative Research and Development Agreement between Rigel and Neurocrine Biosciences, Inc., dated December 1997. | |
| 10.11(1)(2) | Employment Agreement between Rigel and Donald Payan, dated January 16, 1997. | |
63
| 10.13(1) | Technology Transfer Agreement between Rigel and Questcor Pharmaceuticals, Inc., dated September 22, 2000. | |
| 10.14(3)(4) | License and Research Agreement (Amended and Restated) between Rigel and Cell Genesys, Inc., dated September 2, 1999, as amended and restated on March 26, 2001. | |
| 10.15(5) | Lease termination agreement between Rigel and Brittannia Pointe Grand Limited Partnership, dated May 6, 2001. | |
| 10.16(5) | Build-to-suit lease between Rigel and Slough BTC, LLC, dated May 16, 2001. | |
| 10.17(5) | First amendment to the Collaboration Agreement between Rigel and Novartis Pharma AG, dated May 18, 2001. | |
| 10.18(3)(6) | Second Amendment to the Collaboration Agreement between Rigel and Novartis Pharma AG, dated July 6, 2001. | |
| 10.19(3)(6) | Second Amendment to the Collaboration Agreement between Rigel and Cell Genesys, Inc., dated July 1, 2001 | |
| 10.20(2)(11) | 2001 Non-Officer Equity Incentive Plan, as amended. | |
| 10.21(2)(7) | Form of Stock Option Agreement pursuant to the 2001 Non-Officer Equity Incentive Plan. | |
| 10.22(8) | First Amendment to the Collaboration Agreement by and between Rigel and Janssen Pharmaceutica N.V., dated June 30, 2000. | |
| 10.23(8) | Second Amendment to the Collaboration Agreement by and between Rigel and Janssen Pharmaceutica N.V., dated December 4, 2001. | |
| 10.24(10) | Loan and Security Agreement between Rigel and Comerica Bank—California, dated July 12, 2002. | |
| 10.25(10)(3) | Collaboration Agreement between Rigel and Daiichi Pharmaceutical Co., Ltd., dated August 1, 2002. | |
| 10.26(11)(3) | Amendment to Build-to-suit lease between Rigel and Slough BTC, LLC, dated October 18, 2002. | |
| 10.27(11) | Master Lease Agreement between Rigel and Lighthouse Capital Partners IV, L.P., dated December 23, 2002. | |
| 10.28(2)(11) | Employment Agreement between Rigel and Elliott B. Grossbard, dated as of March 18, 2002. | |
| 10.29(2)(11) | Amendment to Employment Agreement between Rigel and Donald Payan, dated as of March 5, 2003. | |
| 23.1(12) | Consent of Ernst & Young LLP, Independent Auditors. | |
| 24.1 | Power of Attorney (included on signature page). | |
| 31.1(12) | Certification required by Rule 13a-14(a) or Rule 15d-14(a) | |
| 31.2(12) | Certification required by Rule 13a-14(a) or Rule 15d-14(a) | |
| 32.1(13) | Certification required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. 1350). |
64
See Item 15(a) above
See Item 15(a) above
65
Pursuant to the requirements of the Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of South San Francisco, State of California, on March 12, 2004.
| Rigel Pharmaceuticals, Inc. | |||
By: |
/s/ JAMES M. GOWER James M. Gower Chairman of the Board and Chief Executive Officer |
||
By: |
/s/ JAMES H. WELCH James H. Welch Vice President, Chief Financial Officer and Secretary |
||
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints James M. Gower and James H. Welch, and each of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him and in his name, place, and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming that all said attorneys-in-fact and agents, or any of them or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated:
| Signature |
Title |
Date |
||
|---|---|---|---|---|
/s/ JAMES M. GOWER James M. Gower |
Chairman of the Board, Chief Executive Officer and Director (Principal Executive Officer) |
March 12, 2004 |
||
/s/ JAMES H. WELCH James H. Welch |
Vice President, Chief Financial Officer, and Secretary (Principal Finance and Accounting Officer) |
March 12, 2004 |
||
/s/ DONALD G. PAYAN Donald G. Payan |
Executive Vice President, Chief Scientific Officer and Director |
March 12, 2004 |
||
/s/ JEAN DELEAGE Jean Deleage |
Director |
March 12, 2004 |
||
66
/s/ ALAN D. FRAZIER Alan D. Frazier |
Director |
March 12, 2004 |
||
/s/ DENNIS J. HENNER Dennis J. Henner |
Director |
March 12, 2004 |
||
/s/ WALTER H. MOOS Walter H. Moos |
Director |
March 12, 2004 |
||
/s/ HOLLINGS C. RENTON Hollings C. Renton |
Director |
March 12, 2004 |
||
/s/ STEPHEN A. SHERWIN Stephen A. Sherwin |
Director |
March 12, 2004 |
||
/s/ NICHOLAS J. SIMON, III Nicholas J. Simon, III |
Director |
March 12, 2004 |
67
| 3.1(1) | Amended and Restated Certificate of Incorporation. | |
| 3.2(1) | Amended and Restated Bylaws. | |
| 4.1(1) | Specimen Common Stock Certificate. | |
| 4.2(1) | Amended and Restated Investor Rights Agreement, between Rigel and holders of Rigel's Series B, Series C, Series D and Series E preferred stock, dated February 3, 2000. | |
| 4.3(1) | Form of warrant to purchase shares of common stock. | |
| 4.7(11) | Amended and Restated Warrant issued to Kwacker Limited for the purchase of shares of common stock. | |
| 4.8(7) | Warrant issued to TBCC Funding Trust II for the purchase of shares of common stock. | |
| 4.9(8) | Warrant issued to Comerica Bank-California for the purchase of shares of common stock | |
| 4.10(11) | Warrant issued to Kwacker Limited for the purchase of shares of common stock. | |
| 4.11(11) | Warrant issued to Lighthouse Capital Partners IV, L.P. to purchase shares of common stock. | |
| 4.12(12) | Warrant issued to Alta BioPharma Partners II, L.P. to purchase shares of common stock. | |
| 4.13(12) | Warrant issued to Alta California Partners, L.P. to purchase shares of common stock. | |
| 4.14(12) | Warrant issued to Alta Embarcadero BioPharma Partners II, LLC to purchase shares of common stock. | |
| 4.15(12) | Warrant issued to Alta Embarcadero Partners, LLC to purchase shares of common stock. | |
| 4.16(12) | Warrant issued to HBM BioVentures (Cayman) Ltd. to purchase shares of common stock. | |
| 4.17(12) | Warrant issued to MPM BioVentures III, L.P. to purchase shares of common stock. | |
| 4.18(12) | Warrant issued to MPM BioVentures III-QP, L.P. to purchase shares of common stock. | |
| 4.19(12) | Warrant issued to MPM BioVentures III GmbH & Co. Beteiligungs KG to purchase shares of common stock. | |
| 4.20(12) | Warrant issued to MPM BioVentures III Parallel Fund, L.P. to purchase shares of common stock. | |
| 4.21(12) | Warrant issued to MPM Asset Management Investors 2003 BVIII LLC to purchase shares of common stock. | |
| 4.22(12) | Warrant issued to MPM BioEquities Master Fund, L.P. to purchase shares of common stock. | |
| 4.23(12) | Second Investor Rights Agreement between Rigel and certain investors, dated June 26, 2003. | |
| 10.1(1) | Form of Indemnity Agreement. | |
| 10.2(11)(2) | 2000 Equity Incentive Plan, as amended. | |
| 10.3(1)(2) | Form of Stock Option Agreement pursuant to 2000 Equity Incentive Plan. | |
| 10.4(1)(2) | 2000 Employee Stock Purchase Plan. | |
| 10.5(1)(2) | 2000 Non-Employee Directors' Stock Option Plan. | |
| 10.6(1) | Collaboration Agreement between Rigel and Janssen Pharmaceutica N.V., dated December 4, 1998. | |
| 10.7(1) | Collaborative Research and License Agreement between Rigel and Pfizer Inc., dated January 31, 1999. | |
| 10.8(1) | Collaboration Agreement between Rigel and Novartis Pharma AG, dated May 26, 1999. | |
| 10.9(1)(3) | License and Research Agreement between Rigel and Cell Genesys, Inc., dated September 2, 1999. | |
| 10.10(1) | Collaborative Research and Development Agreement between Rigel and Neurocrine Biosciences, Inc., dated December 1997. | |
| 10.11(1)(2) | Employment Agreement between Rigel and Donald Payan, dated January 16, 1997. | |
| 10.13(1) | Technology Transfer Agreement between Rigel and Questcor Pharmaceuticals, Inc., dated September 22, 2000. | |
| 10.14(3)(4) | License and Research Agreement (Amended and Restated) between Rigel and Cell Genesys, Inc., dated September 2, 1999, as amended and restated on March 26, 2001. | |
| 10.15(5) | Lease termination agreement between Rigel and Britannia Pointe Grand Limited Partnership, dated May 6, 2001. | |
| 10.16(5) | Build-to-suit lease between Rigel and Slough BTC, LLC, dated May 16, 2001. | |
| 10.17(5) | First Amendment to the Collaboration Agreement between Rigel and Novartis Pharma AG, dated May 18, 2001. | |
| 10.18(3)(6) | Second Amendment to the Collaboration Agreement between Rigel and Novartis Pharma AG, dated July 6, 2001. | |
| 10.19(3)(6) | Second Amendment, dated July 1, 2001, to the Collaboration Agreement between Rigel and Cell Genesys, Inc. | |
| 10.20(2)(11) | 2001 Non-Officer Equity Incentive Plan, as amended. | |
| 10.21(2)(7) | Form of Stock Option Agreement pursuant to the 2001 Non-Officer Equity Incentive Plan. | |
| 10.22(8) | First Amendment to the Collaboration Agreement by and between Rigel and Janssen Pharmaceutical N.V., dated June 30, 2000. | |
| 10.23(8) | Second Amendment to the Collaboration Agreement by and between Rigel and Janssen Pharmaceutical N.V., dated December 4, 2001. | |
| 10.24(10) | Loan and Security Agreement between Rigel and Comerica Bank—California, dated July 12, 2002. | |
| 10.25(10)(3) | Collaboration Agreement between Rigel and Daiichi Pharmaceutical Co., Ltd., dated August 1, 2002. | |
| 10.26(11)(3) | Amendment to Build-to-suit lease between Rigel and Slough BTC, LLC, dated October 18, 2002. | |
| 10.27(11) | Master Lease Agreement between Rigel and Lighthouse Capital Partners IV, L.P., dated December 23, 2002. | |
| 10.28(2)(11) | Employment Agreement between Rigel and Elliott B. Grossbard, dated as of March 18, 2002. | |
| 10.29(2)(11) | Amendment to Employment Agreement, between Rigel and Donald Payan, dated as of March 5, 2003. | |
| 23.1(12) | Consent of Ernst & Young LLP, Independent Auditors. | |
| 24.1 | Power of Attorney (included on signature page). | |
| 31.1(12) | Certification required by Rule 13a-14(a) or Rule 15d-14(a) | |
| 31.2(12) | Certification required by Rule 13a-14(a) or Rule 15d-14(a) | |
| 32.1(13) | Certification required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. 1350). |