UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| (Mark One) | |
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2004 |
|
or |
|
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number 0-29889
RIGEL PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
94-3248524 (IRS Employer Identification Number) |
|
1180 Veterans Blvd. South San Francisco, California (Address of principal executive offices) |
94080 (Zip Code) |
|
(650) 624-1100 (Registrant's telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: None Securities registered pursuant to Section 12(g) of the Act: Common Stock, par value $0.001 per share (Title of Class) |
||
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by a check mark whether the registrant is an accelerated filer (as defined in Exchange Act Rule 12b-2) Yes ý No o
The approximate aggregate market value of the Common Stock held by non-affiliates of the registrant, based upon the closing price of the registrant's Common Stock as reported on the Nasdaq National Market on June 30, 2004, the last business day of the registrant's most recently completed second fiscal quarter, was $175,394,000. Shares of the registrant's outstanding Common Stock held by each executive officer, director and holder of 5% or more of the registrant's outstanding Common Stock have been excluded. The determination of affiliate status for the purposes of this calculation is not necessarily a conclusive determination for other purposes.
As of February 28, 2005, there were 19,670,283 shares of the registrant's Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Items 10, 11, 12, 13 and 14 of Part III incorporate information by reference from the definitive proxy statement for the Registrant's Annual Meeting of Stockholders to be held on June 2, 2005.
| |
|
Page |
|||
|---|---|---|---|---|---|
| PART I | |||||
Item 1. |
Business |
1 |
|||
Item 2. |
Properties |
24 |
|||
Item 3. |
Legal Proceedings |
24 |
|||
Item 4. |
Submission of Matters to a Vote of Security Holders |
24 |
|||
PART II |
|||||
Item 5. |
Market for the Registrant's Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities |
25 |
|||
Item 6. |
Selected Financial Data |
26 |
|||
Item 7. |
Management's Discussion and Analysis of Financial Condition and Results of Operations |
27 |
|||
Item 7A. |
Quantitative and Qualitative Disclosures About Market Risk |
36 |
|||
Item 8. |
Financial Statements and Supplementary Data |
37 |
|||
Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
60 |
|||
Item 9A. |
Controls and Procedures |
60 |
|||
Item 9B. |
Other Information |
61 |
|||
PART III |
|||||
Item 10. |
Directors and Executive Officers of the Registrant |
62 |
|||
Item 11. |
Executive Compensation |
62 |
|||
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
62 |
|||
Item 13. |
Certain Relationships and Related Transactions |
62 |
|||
Item 14. |
Principal Accounting Fees and Services |
62 |
|||
PART IV |
|||||
Item 15. |
Exhibits, Financial Statement Schedules |
63 |
|||
Signatures |
66 |
||||
FORWARD-LOOKING STATEMENTS
This annual report on Form 10-K, including the documents that we incorporate by reference, contains statements indicating expectations about future performance and other forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that involve risks and uncertainties. We usually use words such as "may," "will," "should," "expect," "plan," "anticipate," "believe," "estimate," "predict," "future," "intend," "potential" or "continue" or the negative of these terms or similar expressions to identify these forward-looking statements. These statements appear throughout this annual report on Form 10-K and are statements regarding our current intent, belief or expectation, primarily with respect to our operations and related industry developments. Examples of these statements include, but are not limited to, statements regarding the following: our business and scientific strategies; the progress of our product development programs, including clinical testing; our corporate collaborations, including revenues received from these collaborations; our drug discovery technologies; our research and development expenses; protection of our intellectual property; sufficiency of our cash resources; and our operations and legal risks. You should not place undue reliance on these forward-looking statements. Our actual results could differ materially from those anticipated in these forward-looking statements for many reasons. Any forward-looking statement speaks only as of the date on which it is made, and we undertake no obligation to update any forward- looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
Overview
Rigel Pharmaceuticals, Inc. was incorporated in Delaware in June 1996, and is based in South San Francisco. We discover and develop novel, small-molecule compounds for the treatment of large, unmet medical needs. Our objective is to create a portfolio of product candidates that we will develop for our own proprietary programs and with potential collaborative partners. Our productive discovery engine enables us to move one product candidate into the clinic each year. Currently, we have product development programs for the indications of allergy/asthma, rheumatoid arthritis, cancer, and hepatitis C.
Within the last year, we:
1
Our Strategy
Our objective is to create a portfolio of product candidates that can be developed into small molecule therapeutics for our own proprietary programs and with potential collaborative partners. We believe that producing a portfolio of many product candidates and working in conjunction with pharmaceutical companies increases our probability of development and commercial success. The product development process is one that is subject to both high costs and high risk of failure. We believe that this approach helps minimize the risk of failure, while concurrently strategically placing us in a position to help fill the continuing product pipeline gap at major pharmaceutical companies.
The key elements to our scientific and business strategy are to:
Our discovery engine is based on advanced, proprietary techniques that allow us to identify targets with a demonstrable role in a disease pathway and to screen efficiently for those targets that are likely to be amenable to drug modulation. With this approach, we can generate a portfolio of potential product candidates.
Clinical and Preclinical Product Development Programs
We conduct research and development programs for our product candidates. All of the following programs are owned entirely by us with the exception of our Asthma program, which we licensed to Pfizer in January 2005. We are currently developing several proprietary product candidates. Our most
2
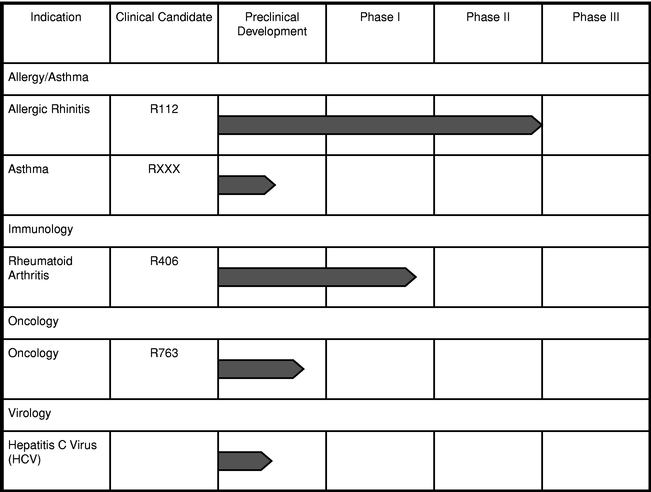
advanced development efforts are described below. The following table summarizes the current status of our proprietary clinical development programs by potential therapeutic indication:

Allergy/Asthma
Disease background. Allergic rhinitis and asthma are chronic inflammatory disorders of the airways. Allergic rhinitis, or allergy, is an acute inflammatory reaction in the upper respiratory tract resulting in nasal congestion, sneezing, itching and watery eyes. Asthma affects the lower respiratory tract and is marked by episodic flare-ups, or attacks, that can be life threatening. In some patients, allergens, such as pollen, trigger the production of immunoglobulin E antibodies, or IgE antibodies, which then bind to mast cells and cause an intracellular signal that results in the release of various chemical mediators. When this process occurs repeatedly over time, it creates persistent inflammation of the airway passages, resulting in the chronic congestion and airway obstruction associated with allergic rhinitis and asthma, respectively. Over 59 million people in the United States suffer from allergic disorders, and over 11 million people suffer from asthmatic disorders.
3
Allergic rhinitis program. R112, our clinical candidate for allergic rhinitis, is an intranasal inhibitor of Syk, or spleen tyrosine kinase, a novel drug target for respiratory diseases such as allergic rhinitis and asthma. Syk is involved in IgE signaling in mast cells. Mast cells play important roles in both early and late phase allergic reactions, and Syk inhibitors could prevent both phases. We completed a Phase I clinical trial of R112 in December 2002 and a single-dose Phase I/II clinical trial in June 2003 that showed R112 was well tolerated and demonstrated physiological responses, including significant statistical improvement or consistent positive trends in reducing the release of chemical mediators involved in mast cell activation, one of the earliest steps in the initiation of an inflammatory response in allergy and asthma. A multi-dose safety trial completed in December 2003 indicated that R112 is well tolerated and demonstrates a favorable safety profile in the study population.
We completed a Phase II "park study" clinical trial of R112 in August 2004. This randomized, placebo-controlled Phase II "park study" enrolled 319 patients with the primary objective of measuring the safety and efficacy of R112 as an intranasal treatment for allergic rhinitis. The "park study" results demonstrated that R112 can reduce certain symptoms of allergic rhinitis in a statistically significant manner compared to placebo, has a favorable safety profile, and an onset of action of approximately thirty minutes. There were no significant drug-related adverse events reported in the trial, and adverse event frequencies were indistinguishable from placebo. As early as the 30-minute time interval after dosing, R112 showed a statistically significant improvement in symptom scores over placebo, demonstrating a rapid onset of action in symptom improvement. Furthermore, these beneficial effects lasted throughout the entire measurement period until the end of the park day. In particular, symptoms most closely associated with chronic nasal congestion (e.g. stuffy nose) were dramatically improved with R112 over placebo.
Based on the results of the single and multi-dose trials as well as the Phase II "park study", we plan to move R112 forward in clinical development with an additional Phase II study expected. We expect this study to be completed later in 2005. We are also actively seeking to partner with a pharmaceutical company with respect to R112. Under terms of an agreement with Pfizer Inc., Pfizer has a limited right of negotiation for R112 under certain circumstances, but the agreement does not preclude us from partnering with other pharmaceutical companies with respect to R112.
Asthma program. In the first quarter of 2005, we announced that we entered into a collaborative research and license agreement with Pfizer for the development of inhaled products for the treatment of allergic asthma and other respiratory diseases, such as COPD. The collaboration is focused on our preclinical small molecule compounds, which inhibit IgE receptor signaling in respiratory tract mast cells by blocking the signaling enzyme Syk kinase.
We expect Pfizer to advance a compound to the clinic combining their dry powder inhaler, their drug development capabilities and our novel small molecules. The first significant milestone under this collaboration is Pfizer's selection of a specific molecule to take into drug development.
Rheumatoid Arthritis
Disease background. Rheumatoid arthritis, or RA, is a chronic inflammatory disease that affects multiple tissues, but typically produces its most pronounced symptoms in the joints. It is often progressive and debilitating, preventing people from living a symptom-free life. Ultimately the chronic inflammation of joints leads to the destruction of the soft tissue and erosion of the articular surfaces of the bone. The disease is estimated to affect nearly 2.1 million people in the United States.
The current treatment options for RA have significant potential side effects and other shortfalls, including gastrointestinal complications and kidney damage. RA patients receive multiple drugs depending on the extent and aggressiveness of the disease. Most RA patients eventually require some form of disease modifying anti-rheumatic drug or DMARD. DMARD's include methotrexate, an
4
anti-cancer agent and Enbrel®, a TNF-blocking agent. TNF-blocking agents inhibit the inflammatory mediator, TNF, and are all delivered via injection.
Rheumatoid arthritis program. We intend to focus our RA program towards the development of an oral, safe DMARD that can be used earlier in the course of the disease, preventing its progression prior to major bone and cartilage destruction. We have selected R406 as our lead product candidate for initial clinical trials in RA. R406 is a novel, oral syk kinase inhibitor that, in preclinical studies, blocks the activation of mast cells and B cells that promote the swelling and inflammatory response. Data from preclinical studies indicate that R406 is effective in a rodent arthritis model, and was without significant toxicity at doses well above the effective dose. We initiated a Phase I clinical trial of R406 in December 2004. The goal of this trial is to establish the safety and pharmacokinetics of R406. The escalating single-dose, placebo-controlled clinical human safety trial included 35 volunteers and is being followed by a multiple-dose study including an additional 24 people. Results of the trial are expected within the next few months and, if favorable, will, allow us to enter broader, longer-term safety and efficacy trials in patients suffering from rheumatoid arthritis.
Oncology
Disease background. Cancer is the second leading cause of death in the United States. More than one million people get cancer each year, and nearly half of all men and a little over one-third of all women in the United States will develop cancer during their lifetimes. Anyone can get cancer at any age, however, approximately 77% of all cancers are diagnosed in people age 55 and older. Although cancer occurs in all racial and ethnic groups, the rate of incidence varies from group to group.
Aurora Kinase program. Aurora kinase plays a central role in the cell division process and the overexpression of aurora kinase can cause cells to quickly form an abnormal number of chromosomes. As such, aurora kinase is frequently associated with various solid tumor human cancers such as cancers of the breast, bladder, colon, ovary, head and neck, and pancreas. Increased knowledge of aurora kinase and its regulation potential may be the basis for treating and even preventing cancer.
R763 is a potent, highly-selective, small-molecule inhibitor of aurora kinase. In July 2004, we identified R763 as a lead compound in our aurora kinase inhibition program, targeting cancer cell proliferation. We intend to file an IND in 2005.
Hepatitis C Virus
Disease background. Hepatitis C is an inflammation of the liver caused by the hepatitis C virus. As the most common chronic blood-borne infection in the United States, the hepatitis C virus, or HCV, affects an estimated 3.9 million people in the United States and 170 million individuals worldwide. Approximately 80% of those with an acute illness will develop chronic hepatitis, a condition that has been linked to cirrhosis, liver failure and hepatocellular carcinoma, or liver cancer. HCV is a leading cause of chronic liver disease and is the most common indication for liver transplantation.
Currently available HCV therapies are only modestly effective at treating the disease. The most prevalent treatment regimen is with interferon alpha, or IFN, or its longer lasting pegylated version, usually in combination with ribavarin. IFN therapy works to boost the body's own immune system and generally requires six to 12 months of therapy to be effective. Only 20% to approximately 40% of the patients who complete IFN therapy have a successful response. IFN dosage must be reduced in 10% to 40% of patients and discontinued in 5% to 15% of patients because of severe side effects. Moreover, IFN is least effective against HCV genotype 1, the strain responsible for approximately 70% of chronic HCV cases in the United States.
Anti-HCV program. Our lead program addressing HCV has identified various compounds that are oral small molecules that work directly, rapidly and selectively on the virus by interfering with a viral
5
polymerase protein that is needed for replication. The first of these product candidates to enter the clinic was R803 with the completion of a Phase I/II study and announcement of the results in November 2004. Based on the clinical trial results of this study, we made the decision not to move forward with R803. However, we are aggressively examining alternative compounds for selection of a candidate to move forward into the clinic. Upon completion of this evaluation, we will be able to articulate our future course of action. We expect to reach a determination as to whether any of these alternative compounds meets our criteria by the middle of 2005.
Research Programs
We are conducting proprietary research in three broad disease areas: immunology/inflammation, virology and oncology. With each disease area we are conducting basic research as well as screening compounds against potential novel intracellular targets and optimizing those leads that appear most promising.
Currently, we are researching autoimmune mediated inflammation disorders such as transplant rejection, multiple sclerosis and inflammation of the bowel. We have identified more than one kinase that may be inhibited in order to treat inflammation related disorders, and we are in the process of screening other compounds against various kinases in order to find additional lead compounds to potentially treat inflammation related disorders. In the area of virology, we are investigating other potential targets to inhibit HCV replication. In addition, we are conducting initial screening tests of potential product candidates against other viruses. In the area of oncology, we are focused on inhibiting kinases as well as ligases (see below), a new target class that also may yield possible drug targets in immunology and virology as well.
Ubiquitin ligase program. Ubiquitin ligases are enzymes that regulate protein degradation within the cell. The breakdown of proteins, in turn, affects many important cellular functions, including cell division. Targeting ligases represents a novel approach to treating diseases where normal cellular processes are out of balance. Because unchecked cell division is the hallmark of cancer, researchers believe that this part of the cell machinery represents a particularly compelling target for cancer therapies. Ubiquitin ligase targets are numerous and modular. This provides the potential for intervening in a highly specific fashion with respect to a disease, potentially improving efficacy and minimizing side-effects.
We believe we are a leader in investigating and characterizing the ubiquitin ligase system for the discovery and development of potential new therapeutics in the oncology as well as immunology and virology areas. We have initiated one of the industry's broadest efforts with respect to ubiquitin ligase, working on the development of numerous ligase targets, and were one of the first companies to discover potent and highly selective small molecule inhibitors of ubiquitin ligases. Some of these inhibitors have shown positive activity in animal models of disease and are part of our preclinical development program.
In November 2004, we entered into a broad collaboration agreement with Merck and Co., Inc. to investigate ubiquitin ligases to find treatments for cancer and potentially other diseases. The collaboration is based on a number of new targets designated by Merck and do not include our current ligase targets. However, we may nominate our own targets for potential inclusion in the collaboration. We also have an ongoing program with Daiichi to pursue research related to ubiquitin ligases.
Corporate Collaborations
Current Collaborations
In addition to the preceding programs, we also carry on research and development programs in connection with our corporate collaborations. With the exception of our asthma program, for which we
6
recently partnered with Pfizer, we retain economic and commercial rights for all of the clinical and preclinical product development programs described above. We currently have collaborations with five major pharmaceutical companies, including one with Janssen Pharmaceutica N.V., a division of Johnson & Johnson, relating to oncology therapeutics and diagnostics, two with Pfizer relating to programs in asthma and allergy therapeutics, one with Novartis Pharma AG regarding four different programs relating to immunology, oncology and chronic bronchitis, one with Daiichi Pharmaceuticals Co., Ltd. in the area of oncology, and one with Merck to investigate ubiquitin ligases with the goal of finding treatments for cancer and potentially other diseases. These collaborations all have or had a research phase during which we receive or received funding based on the level of headcount allocated to a program. In all of our collaborations, after the research phase concludes and if certain conditions are met, we are entitled to receive future milestone payments and royalties. Only the Daiichi and Merck programs are currently in the research phase of their respective agreements and provide for regular research reimbursement payments.
Daiichi
In August 2002, we entered into an agreement with Daiichi to pursue research related to ligases, a novel class of drug targets that control cancer cell proliferation through protein degradation. Through this collaboration, we are working with Daiichi to discover and develop cancer pharmaceutical drugs. The initial stages of the Daiichi collaboration focused on the development of the assay for the specific target and the initiation of high-throughput compound screening to identify therapeutic molecules we and Daiichi would like to advance to later stages of product development. Under terms of the agreement, we retain the rights to co-develop and co-promote products resulting from this collaboration in North America while Daiichi retains co-development and promotion rights in the remainder of the world. Per the agreement, the research phase of this collaboration is set to expire in August 2005. Prior to the expiration of the research phase, Daiichi is obligated to pay us ongoing research support and may become obligated to pay us certain other milestones payments. In addition, we are entitled to receive royalties on any commercialized products to emerge from the collaboration.
Johnson & Johnson
Effective December 1998, we entered into a three-year research collaboration with Janssen Pharmaceutica N.V., a division of Johnson & Johnson, which was extended through December 2003, to identify, discover and validate novel drug targets that regulate cell cycle, and, specifically, to identify drug targets and the active peptides that bind to them that can restore a mutated cell's ability to stop uncontrolled cell division. Under the agreement, we provided certain assays and associated technology to Johnson & Johnson for the assessment of the alteration or normalization of the dysfunctional cell cycles of cancer cells for Johnson & Johnson's internal research purposes. We entered into an amendment in July 2000, which expanded the collaboration by having us perform compound screening and medicinal chemistry on some of the validated targets accepted by Johnson & Johnson. We have identified several novel drug targets in this program, nine of which have been accepted by Johnson & Johnson as validated. Two of these nine targets have completed high-throughput screening, or HTS, at our facilities. Johnson & Johnson continues to be obligated to pay us various milestones and royalties if certain conditions are met.
Merck
In November 2004, we entered into a broad collaboration agreement with Merck to investigate ubiquitin ligases, a new class of drug target, to find treatments for cancer and potentially other diseases. Under the terms of the agreement, we received an initial cash payment and will receive funding for our research scientists for two and a half years, at which point the research phase of this collaboration will terminate. The collaboration is based on a number of new targets designated by
7
Merck and do not include our current ligase targets. In addition, we may nominate our own targets for potential inclusion in the collaboration. Merck is responsible for worldwide development and commercialization of any resulting compounds and will pay us royalties on future product sales, if any. Under this collaboration, if certain conditions are met, we are eligible to receive milestone payments for preclinical and clinical events.
Novartis
In May 1999, we entered into an agreement for the establishment of a broad collaboration with Novartis. We agreed to work with Novartis on up to five different five-year research projects to identify drug targets for products that can treat, prevent or diagnose the effects of human disease. Two of the research projects were conducted jointly by Novartis and us, and the other three research projects were to be conducted at Novartis. The first research project, a joint research project, focused on identifying small molecule drug targets that regulate T cells in the area of transplant rejection. The second research project, also a joint research project, related to the identification and validation of small molecule drug targets that mediate specific functions of B cells in the area of autoimmunity. Pursuant to the collaboration agreement, Novartis had the option to end the research phase on these programs after either 24 months or 42 months. In May 2002, Novartis elected to conclude the research phases of our two initial joint projects in the autoimmunity and transplant rejection areas after 42 months each, effective in November 2002 and February 2003, respectively. The third research project, a project currently being carried out at Novartis, is focused on identifying small molecule drug targets that regulate chronic bronchitis. Novartis may terminate this chronic bronchitis research at any time. In July 2001, we amended the agreement to add a three-year joint project at our facilities in the area of angiogenesis in lieu of one of the projects that were to be conducted at Novartis. As a result of this amendment, Novartis provided funding for research that was conducted at our facilities and made an additional upfront payment. In January 2002, Novartis chose not to exercise its option to add a final project that was to be conducted at Novartis. Novartis continues to be obligated to pay us various milestones and royalties if certain conditions are met.
Pfizer
Effective January 1999, we entered into a research collaboration with Pfizer to identify and validate intracellular drug targets that control and inhibit the production of IgE in B-cells in the area of asthma/allergy. The research phase of the collaboration was initially scheduled to end on January 31, 2001. In January 2001, Pfizer notified us of its election to exercise its option to extend the funded research portion of the collaboration one additional year to January 31, 2002. During the research phase, the collaboration was successful in identifying several intracellular drug targets that control the production of IgE, a key mediator in allergic reactions and asthma in B-cells. Through the conclusion of the research phase of the collaboration, which was extended by one additional month to February 28, 2002, Pfizer accepted a total of seven validated targets. Pfizer continues to be obligated to pay us various milestones and royalties if certain conditions are met.
In January 2005, we entered into a second research collaboration with Pfizer that has a license component. The collaboration is for the development of inhaled products for the treatment of allergic asthma and other respiratory diseases such COPD. The collaboration is focused on our preclinical small molecule compounds, which inhibit IgE receptor signaling in respiratory tract mast cells by blocking the signaling enzyme Syk kinase. The Syk kinase intrapulmonary collaboration with Pfizer does not include R112 our lead Syk kinase inhibitor that is being developed for the treatment of allergic rhinitis or the right to develop any of our Syk inhibitors in the field of allergic rhinitis. In August 2004, we completed a successful Phase II clinical study with R112 and are proceeding with the further clinical development of R112 for allergic rhinitis. Under certain conditions, Pfizer has a limited option to negotiate a license to R112 and our Syk inhibitors in the allergic rhinitis field under different financial and other terms
8
and conditions than are provided for in the current collaboration. Pursuant to the terms of the current collaboration, we received an upfront cash payment, and are eligible to receive milestone payments and royalties on any future product sales. Pfizer made an equity investment in Rigel at a premium and will be responsible for the worldwide development and commercialization of any resulting products.
Our Discovery Engine
The technologies that we use in connection with both our proprietary product development programs and our corporate collaborations are designed to identify protein targets for compound screening and validate the role of those targets in the disease process. Unlike genomics-based approaches, which begin by identifying genes and then search for their functions, our approach identifies proteins that are demonstrated to have an important role in a disease pathway. By understanding the disease pathway, we attempt to avoid studying genes that will not make good drug targets and focus only on the sub set of expressed proteins of genes that we believe are specifically implicated in the disease process.
We begin by developing assays that model the key events in a disease process at the cellular level. We then efficiently search hundreds of millions of cells to identify potential protein targets. In addition, we identify the proteins involved in the intracellular process and prepare a map of their interactions, thus giving us a comprehensive picture of the intracellular disease pathway. We believe that our approach has a number of advantages, including:
Because of the very large number of cells and proteins employed, our technology is labor intensive. The complexity of our technology requires a high degree of skill and diligence to perform successfully. In addition, successful application of our technology depends on a highly diverse collection of proteins to test in cells. We believe we have been able to and will continue to meet these challenges successfully and increase our ability to identify targets for drug discovery. Although other companies may utilize technologies similar to certain aspects of our technology, we are unaware of any other company that employs the same combination of technologies as we do.
Pharmacology and Preclinical Development
We believe that the rapid characterization and optimization of lead compounds identified in HTS will generate high-quality preclinical development candidates. Our pharmacology and preclinical
9
development group facilitates lead optimization by characterizing lead compounds with respect to pharmacokinetics, potency, efficacy and selectivity. The generation of proof-of-principle data in animals and the establishment of standard pharmacological models with which to assess lead compounds represent integral components of lead optimization. As programs move through the lead optimization stage, our pharmacology and preclinical development group supports our chemists and biologists by performing the necessary studies, including toxicology, for investigational new drug, or IND application submissions.
Clinical Development
We have assembled a team of experts in drug development to design and implement clinical trials and to analyze the data derived from these studies. The clinical development group possesses expertise in project management and regulatory affairs.
Research and Development Expenses
Our research and development expenses were $48.5 million in 2004, $41.6 million in 2003 and $40.8 million in 2002.
Intellectual Property
We are able to protect our technology from unauthorized use by third parties only to the extent that it is covered by valid and enforceable patents or is effectively maintained as a trade secret. Accordingly, patents or other proprietary rights are an essential element of our business. We have over 150 pending patent applications and over 50 issued patents in the United States that are owned by or exclusively licensed to us in our field as well as pending corresponding foreign patent applications. Our policy is to file patent applications to protect technology, inventions and improvements to inventions that are commercially important to the development of our business. We seek United States and international patent protection for a variety of technologies, including new screening methodologies and other research tools, target molecules that are associated with disease states identified in our screens, and lead compounds that can affect disease pathways. We also intend to seek patent protection or rely upon trade secret rights to protect other technologies that may be used to discover and validate targets and that may be used to identify and develop novel drugs. We seek protection, in part, through confidentiality and proprietary information agreements. We are a party to various license agreements that give us rights to use technologies in our research and development.
Competition
We face, and will continue to face, intense competition from pharmaceutical and biotechnology companies, as well as from academic and research institutions and government agencies, both in the United States and abroad. Some of these competitors are pursuing the development of pharmaceuticals that target the same diseases and conditions as our research programs. Our major competitors include fully integrated pharmaceutical companies that have extensive drug discovery efforts and are developing novel small molecule pharmaceuticals. We also face significant competition from organizations that are pursuing the same or similar technologies, including the discovery of targets that are useful in compound screening, as the technologies used by us in our drug discovery efforts. Our competitors or their collaborative partners may utilize discovery technologies and techniques or partner more rapidly or successfully than we or our collaborators are able to do.
Many of these companies and institutions, either alone or together with their collaborative partners, have substantially greater financial resources and larger research and development staffs than
10
we do. In addition, many of these competitors, either alone or together with their collaborative partners, have significantly greater experience than we do in:
Accordingly, our competitors may succeed in obtaining patent protection, identifying or validating new targets or discovering new drug compounds before we do.
Competition may also arise from:
Developments by others may render our product candidates or technologies obsolete or noncompetitive. We face and will continue to face intense competition from other companies for collaborative arrangements with pharmaceutical and biotechnology companies, for establishing relationships with academic and research institutions and for licenses to additional technologies. These competitors, either alone or with their collaborative partners, may succeed in developing technologies or products that are more effective than ours.
Our ability to compete successfully will depend, in part, on our ability to:
Government Regulation
Our ongoing development activities are and will be subject to extensive regulation by numerous governmental authorities in the United States and other countries, including the Food and Drug Administration, or FDA under the Federal Food, Drug and Cosmetic Act. The regulatory review and approval process is expensive and uncertain. Securing FDA approval requires the submission of extensive preclinical and clinical data and supporting information to the FDA for each indication to establish a product candidate's safety and efficacy. The approval process takes many years, requires the expenditure of substantial resources and may involve ongoing requirements for post-marketing studies. Clinical trials are subject to oversight by institutional review boards and the FDA and:
11
Even if we are able to achieve success in our clinical testing, we, or our collaborative partners, must provide the FDA and foreign regulatory authorities with clinical data that demonstrates the safety and efficacy of our products in humans before they can be approved for commercial sale. We also do not know whether any future clinical trials will demonstrate sufficient safety and efficacy necessary to obtain the requisite regulatory approvals or will result in marketable products. Our failure, or the failure of our strategic partners, to adequately demonstrate the safety and efficacy of our products under development will prevent receipt of FDA and similar foreign regulatory approval and, ultimately, commercialization of our products.
Any clinical trial may fail to produce results satisfactory to the FDA. Preclinical and clinical data can be interpreted in different ways, which could delay, limit or prevent regulatory approval. Negative or inconclusive results or adverse medical events during a clinical trial could cause a clinical trial to be repeated or a program to be terminated. In addition, delays or rejections may be encountered based upon additional government regulation from future legislation or administrative action or changes in FDA policy or interpretation during the period of product development, clinical trials and FDA regulatory review. Failure to comply with applicable FDA or other applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production or injunction, as well as other regulatory action against our potential products, collaborative partners or us. Additionally, we have no experience in working with our partners in conducting and managing the clinical trials necessary to obtain regulatory approval.
Outside the United States, our ability to market a product is contingent upon receiving a marketing authorization from the appropriate regulatory authorities. The requirements governing the conduct of clinical trials, marketing authorization, pricing and reimbursement vary widely from country to country. At present, foreign marketing authorizations are applied for at a national level, although within the European Union, or EU, registration procedures are available to companies wishing to market a product in more than one EU member state. If the regulatory authority is satisfied that adequate evidence of safety, quality and efficacy has been presented, a marketing authorization will be granted. This foreign regulatory approval process involves all of the risks associated with FDA clearance.
Employees
As of December 31, 2004, we had 144 employees.
Scientific & Medical Advisors
We utilize scientists and physicians to advise us on scientific and medical matters as part of our ongoing research and product development efforts, including experts in clinical trial design, preclinical development work, chemistry, biology, infectious diseases, immunology and oncology. Certain of our scientific and medical advisors and consultants receive an option to purchase our common stock and an honorarium for time spent assisting us.
12
Available Information
We maintain a site on the world wide web at www.rigel.com. The information found on our website is not incorporated by reference into this annual report on Form 10-K. We electronically file with the Securities and Exchange Commission our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, our director and officers' Section 16 reports, other SEC filings and amendments to the reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. We make available free of charge on or through our website copies of these reports as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission, or SEC. Further, a copy of these reports is located at the Securities and Exchange Commission's Public Reference Room at 450 Fifth Street, NW, Washington, D.C. 20549. Information on the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330. The SEC also maintains an internet site that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC at www.sec.gov.
Risk Factors
In evaluating our business, you should carefully consider the following risks, as well as the other information contained in this annual report on Form 10-K. If any of the following risks actually occurs, our business could be harmed. The risks and uncertainties described below are not the only ones facing us. Additional risks and uncertainties not presently known to us, or that we currently see as immaterial, may also harm our business.
We will need additional capital in the future to sufficiently fund our operations and research.
We have consumed substantial amounts of capital to date, and operating expenditures are expected to increase over the next several years. We believe that our existing capital resources and anticipated proceeds from current collaborations will be sufficient to support our current operating plan through at least the next twelve months. Our operations will require significant additional funding in large part due to our research and development expenses, future preclinical and clinical-testing costs, and the absence of any meaningful revenues for the foreseeable future. The amount of future funds needed will depend largely on the timing and structure of potential future collaborations. We do not know whether additional financing will be available when needed, or that, if available, we will obtain financing on terms favorable to our stockholders or us. We have consumed substantial amounts of capital to date, and operating expenditures are expected to increase over the next several years as we expand our infrastructure and research and development activities.
To the extent we raise additional capital by issuing equity securities, our stockholders would at that time experience substantial dilution. To the extent that we raise additional funds through collaboration and licensing arrangements, we may be required to relinquish some rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us.
Our future funding requirements will depend on many uncertain factors.
Our future funding requirements will depend upon many factors, including, but not limited to:
13
Insufficient funds may require us to delay, scale back or eliminate some or all of our research or development programs, to lose rights under existing licenses or to relinquish greater or all rights to product candidates at an earlier stage of development or on less favorable terms than we would otherwise choose or may adversely affect our ability to operate as a going concern.
Our success as a company is uncertain due to our history of operating losses and the uncertainty of future profitability.
Due in large part to the significant research and development expenditures required to identify and validate new product candidates and pursue our development efforts, we have not been profitable and have incurred operating losses since we were incorporated in June 1996. The extent of our future losses and the timing of potential profitability are highly uncertain, and we may never achieve profitable operations. We incurred net losses of $56.3 million in 2004, $41.2 million in 2003, and $37.0 million in 2002. Currently, our revenues are generated solely from research payments pursuant to our collaboration agreements and licenses and are insufficient to generate profitable operations. As of December 31, 2004, we had an accumulated deficit of approximately $212.3 million. We expect to incur losses for at least the next several years and expect that these losses could increase as we expand our research and development activities and incur significant clinical and testing costs.
There is a high risk that early-stage drug discovery and development might not successfully generate good product candidates.
At the present time, the majority of our operations are in the early stages of drug identification and development. To date, three of our product compounds have made it to the clinical testing stage. In our industry, it is statistically unlikely that the limited number of compounds that we have identified as potential product candidates will actually lead to successful product development efforts, and we do not expect any drugs resulting from our research to be commercially available for several years, if at all. Our product compounds in the clinic and our future leads for potential drug compounds are subject to the risks and failures inherent in the development of pharmaceutical products based on new technologies. These risks include, but are not limited to, the inherent difficulty in selecting the right drug target and avoiding unwanted side effects as well as unanticipated problems relating to product development, testing, regulatory compliance, manufacturing, marketing, competition and costs and expenses that may exceed current estimates. The results of preliminary studies do not necessarily predict clinical or commercial success, and larger later-stage clinical trials may fail to confirm the results observed in the preliminary studies. With respect to our own compounds in development, we have established anticipated timelines for clinical development based on existing knowledge of the
14
compound. However, we cannot provide assurance that we will meet any of these timelines with respect to the initiation or completion of clinical studies.
We have also commenced clinical trials of R406 in December 2004 and expect to initiate clinical trials of R763 in the second half of 2005. Because of the uncertainty of whether the accumulated preclinical evidence (pharmacokinetic, pharmacodynamic, safety and/or other factors) or early clinical results will be observed in later clinical trials, we can make no assurance regarding the likely results from our future clinical trials or the impact of those results on our business.
We might not be able to commercialize our product candidates successfully if problems arise in the clinical testing and approval process.
Commercialization of our product candidates depends upon successful completion of preclinical studies and clinical trials. Preclinical testing and clinical development are long, expensive and uncertain processes. We do not know whether we, or any of our collaborative partners, will be permitted to undertake clinical trials of potential products beyond the trials already concluded and the trials currently in process. It may take us or our collaborative partners several years to complete any such testing, and failure can occur at any stage of testing. Interim results of trials do not necessarily predict final results, and acceptable results in early trials may not be repeated in later trials. A number of companies in the pharmaceutical industry, including biotechnology companies, have suffered significant setbacks in advanced clinical trials, even after achieving promising results in earlier trials. Moreover, as our projects reach clinical trials, we or our collaborative partners or regulators may decide to discontinue development of any or all of these projects at any time for commercial, scientific or other reasons. For example, if patients experience undesirable side effects, we may be required to halt or suspend a clinical trial.
Delays in clinical testing could result in increased costs to us.
Significant delays in clinical testing could materially impact our product development costs. We do not know whether planned clinical trials will begin on time, will need to be revamped or will be completed on schedule, or at all. Clinical trials can be delayed for a variety of reasons, including delays in obtaining regulatory approval to commence a study, delays in reaching agreement on acceptable clinical study agreement terms with prospective clinical sites, delays in obtaining institutional review board approval to conduct a study at a prospective clinical site or delays in recruiting subjects to participate in a study. Environmental conditions may impact the execution of some clinical trials, particularly in the allergy area.
In addition, we typically rely on third-party clinical investigators to conduct our clinical trials and other third-party organizations to oversee the operations of such trials and to perform data collection and analysis. As a result, we may face additional delaying factors outside our control if these parties do not perform their obligations in a timely fashion. While we have not yet experienced delays that have materially impacted our clinical trials or product development costs, delays of this sort could occur for the reasons identified above or other reasons. If we have delays in testing or approvals, our product development costs will increase. For example, we may need to make additional payments to third-party investigators and organizations to retain their services or we may need to pay recruitment incentives. If the delays are significant, our financial results and the commercial prospects for our product candidates will be harmed, and our ability to become profitable will be delayed.
15
We lack the capability to manufacture compounds for development and rely on third parties to manufacture our product candidates, and we may be unable to obtain required material in a timely manner, at an acceptable cost or at a quality level required to receive regulatory approval.
We currently do not have manufacturing capabilities or experience necessary to produce materials, including R112, R406, and R763 for preclinical testing and clinical trials. We rely on a single third-party contractor to produce R112 and R406 bulk drug substance. We also rely on different single manufacturers for finished R112 and R406 product for preclinical and clinical testing. We will rely on manufacturers to deliver materials on a timely basis and to comply with applicable regulatory requirements, including the FDA's current Good Manufacturing Practices, or GMP. These outsourcing efforts with respect to manufacturing preclinical and clinical supplies will result in a dependence on our suppliers to timely manufacture and deliver sufficient quantities of materials produced under GMP conditions to enable us to conduct planned preclinical studies, clinical trials and, if possible, to bring products to market in a timely manner.
Our current and anticipated future dependence upon these third-party manufacturers may adversely affect our ability to develop and commercialize product candidates on a timely and competitive basis. These manufacturers may not be able to produce material on a timely basis or manufacture material at the quality level or in the quantity required to meet our development timelines and applicable regulatory requirements. We may not be able to maintain or renew our existing third-party manufacturing arrangements, or enter into new arrangements, on acceptable terms, or at all. Our third-party manufacturers could terminate or decline to renew our manufacturing arrangements based on their own business priorities, at a time that is costly or inconvenient for us. If we are unable to contract for the production of materials in sufficient quantity and of sufficient quality on acceptable terms, our planned clinical trials may be delayed. Delays in preclinical or clinical testing could delay the filing of our IND applications and/or the initiation of clinical trials that we have currently planned.
Our third-party manufacturers may not be able to comply with the GMP regulations, other applicable FDA regulatory requirements or similar regulations applicable outside of the United States. Additionally, if we are required to enter into new supply arrangements, we may not be able to obtain approval from the FDA of any alternate supplier in a timely manner, or at all, which could delay or prevent the clinical development and commercialization of any related product candidates. Failure of our third-party manufacturers or us to obtain approval from the FDA or to comply with applicable regulations could result in sanctions being imposed on us, including fines, civil penalties, delays in or failure to grant marketing approval of our product candidates, injunctions, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of products and compounds, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect our business.
Because most of our expected future cash proceeds are contingent upon collaborative and license agreements, we might not meet our strategic objectives.
Our ability to generate cash proceeds in the near term depends on our ability to enter into additional collaborative agreements with third parties and to maintain the agreements we currently have in place. Our ability to enter into new collaborations and the cash proceeds, if any, that may be earned under these collaborations is highly uncertain. If we are unable to enter into new collaborations, our business prospects could be harmed, which could have an immediate adverse effect on the trading price of our stock.
To date, most of our cash proceeds have been related to the research phase of each of our collaborative agreements. Such cash proceeds are for specified periods, and the impact of such cash procceds on our results of operations is partially offset by corresponding research costs. Following the completion of the research phase of each collaborative agreement, additional cash proceeds may come only from milestone payments and royalties, which may not be paid, if at all, until some time well into
16
the future. The risk is heightened due to the fact that unsuccessful research efforts may preclude us from receiving any milestone payments under these agreements. Our receipt of cash proceeds from collaborative arrangements is also significantly affected by the timing of efforts expended by us and our collaborators and the timing of lead compound identification. In late 2001, we earned the first cash proceed from achievement of milestones in both the Pfizer and Johnson & Johnson collaborations. During 2002, we earned our first milestone for both Novartis and Daiichi. Under many agreements, however, milestone payments may not be earned until the collaborator has advanced products into clinical testing, which may never occur or may not occur until some time well into the future. If we are not able to generate cash proceeds under our collaborations when and in accordance with our expectations or the expectations of industry analysts, this failure could harm our business and have an immediate adverse effect on the trading price of our stock.
Our business requires us to generate meaningful cash proceeds from royalties and licensing agreements. To date, we have not received any cash proceeds from royalties for the commercial sale of drugs, and we do not know when we will receive any such cash proceeds, if at all. Likewise, we have not licensed any lead compounds or drug development candidates to third parties, and we do not know whether any such license will be entered into on acceptable terms in the future, if at all.
If our current corporate collaborations or license agreements are unsuccessful, our research and development efforts could be delayed.
Our strategy depends upon the formation and sustainability of multiple collaborative arrangements and license agreements with third parties in the future. We rely on these arrangements for not only financial resources, but also for expertise that we expect to need in the future relating to clinical trials, manufacturing, sales and marketing, and for licenses to technology rights. To date, we have entered into several such arrangements with corporate collaborators; however, we do not know if such third parties will dedicate sufficient resources or if any development or commercialization efforts by third parties will be successful. Should a collaborative partner fail to develop or commercialize a compound or product to which it has rights from us, such failure might delay ongoing research and development efforts at Rigel because we might not receive any future milestone payments and we would not receive any royalties associated with such compound or product. In addition, the continuation of some of our partnered drug discovery and development programs may be dependent on the periodic renewal of our corporate collaborations.
The research phase of our collaboration with Johnson & Johnson ended in December 2003 and the research phases conducted at our facilities under our broad collaboration with Novartis ended in July 2004. The research phase of our corporate collaboration agreement with Daiichi will end in August 2005. In November 2004 we signed a new corporate collaboration with Merck and in January 2005 we signed an additional collaboration with Pfizer. We may not be able to renew these collaborations on acceptable terms, if at all, or negotiate additional corporate collaborations on acceptable terms, if at all.
Conflicts also might arise with collaborative partners concerning proprietary rights to particular compounds. While our existing collaborative agreements typically provide that we retain milestone payments and royalty rights with respect to drugs developed from certain derivative compounds, any such payments or royalty rights may be at reduced rates, and disputes may arise over the application of derivative payment provisions to such drugs, and we may not be successful in such disputes.
We are also a party to various license agreements that give us rights to use specified technologies in our research and development processes. The agreements pursuant to which we have in-licensed technology permit our licensors to terminate the agreements under certain circumstances. If we are not able to continue to license these and future technologies on commercially reasonable terms, our product development and research may be delayed.
17
If conflicts arise between our collaborators or advisors and us, any of them may act in their self-interest, which may be adverse to our stockholders' interests.
If conflicts arise between us and our corporate collaborators or scientific advisors, the other party may act in its self-interest and not in the interest of our stockholders. Some of our corporate collaborators are conducting multiple product development efforts within each disease area that is the subject of the collaboration with us. In some of our collaborations, we have agreed not to conduct, independently or with any third party, any research that is competitive with the research conducted under our collaborations. Our collaborators, however, may develop, either alone or with others, products in related fields that are competitive with the products or potential products that are the subject of these collaborations. Competing products, either developed by our collaborators or to which our collaborators have rights, may result in their withdrawal of support for our product candidates.
If any of our corporate collaborators were to breach or terminate its agreement with us or otherwise fail to conduct the collaborative activities successfully and in a timely manner, the preclinical or clinical development or commercialization of the affected product candidates or research programs could be delayed or terminated. We generally do not control the amount and timing of resources that our corporate collaborators devote to our programs or potential products. We do not know whether current or future collaborative partners, if any, might pursue alternative technologies or develop alternative products either on their own or in collaboration with others, including our competitors, as a means for developing treatments for the diseases targeted by collaborative arrangements with us.
Our success is dependent on intellectual property rights held by us and third parties, and our interest in such rights is complex and uncertain.
Our success will depend to a large part on our own, our licensees' and our licensors' ability to obtain and defend patents for each party's respective technologies and the compounds and other products, if any, resulting from the application of such technologies. We have over 150 pending patent applications and over 50 issued patents in the United States that are owned or exclusively licensed in our field as well as pending corresponding foreign patent applications. In the future, our patent position might be highly uncertain and involve complex legal and factual questions. Additional uncertainty may result from because no consistent policy regarding the breadth of legal claims allowed in biotechnology patents has emerged to date. Accordingly, we cannot predict the breadth of claims allowed in our or other companies' patents.
Because the degree of future protection for our proprietary rights is uncertain, we cannot ensure that:
We rely on trade secrets to protect technology where we believe patent protection is not appropriate or obtainable. However, trade secrets are difficult to protect. While we require employees,
18
collaborators and consultants to enter into confidentiality agreements, we may not be able to adequately protect our trade secrets or other proprietary information in the event of any unauthorized use or disclosure or the lawful development by others of such information.
We are a party to certain in-license agreements that are important to our business, and we generally do not control the prosecution of in-licensed technology. Accordingly, we are unable to exercise the same degree of control over this intellectual property as we exercise over our internally-developed technology. Moreover, some of our academic institution licensors, research collaborators and scientific advisors have rights to publish data and information in which we have rights. If we cannot maintain the confidentiality of our technology and other confidential information in connection with our collaborations, then our ability to receive patent protection or protect our proprietary information will be impaired. In addition, some of the technology we have licensed relies on patented inventions developed using U.S. government resources. The U.S. government retains certain rights, as defined by law, in such patents, and may choose to exercise such rights. Certain of our in-licenses may be terminated if we fail to meet specified obligations. If we fail to meet such obligations and any of our licensors exercise their termination rights, we could lose our rights under those agreements. If we lose any of our rights, it may affect the way we do business. In addition, because certain of our licenses are sublicenses, the actions of our licensors may affect our rights under those licenses.
If a dispute arises regarding the infringement or misappropriation of the proprietary rights of others, such dispute could be costly and result in delays in our research and development activities and partnering.
Our success will also depend, in part, on our ability to operate without infringing or misappropriating the proprietary rights of others. There are many issued patents and patent applications filed by third parties relating to products or processes that are similar or identical to ours or our licensors, and others may be filed in the future. There can be no assurance that our activities, or those of our licensors, will not infringe patents owned by others. For example, in June 2002, we resolved a dispute with Inoxell A/S (formed as a spinout from Pharmexa—formally M&E Biotech) by entering into a global patent settlement concerning certain drug target identification technologies, which includes both cross-licensing and joint ownership with respect to certain patents and allows for worldwide freedom of operation for both companies. We believe that there may be significant litigation in the industry regarding patent and other intellectual property rights, and we do not know if we or our collaborators would be successful in any such litigation. Any legal action against our collaborators or us claiming damages or seeking to enjoin commercial activities relating to the affected products, our methods or processes could:
If we are unable to obtain regulatory approval to market products in the United States and foreign jurisdictions, we might not be permitted to commercialize products from our research and development.
Due, in part, to the early stage of our product candidate research and development process, we cannot predict whether regulatory clearance will be obtained for any product that we, or our
19
collaborative partners, hope to develop. Satisfaction of regulatory requirements typically takes many years, is dependent upon the type, complexity and novelty of the product and requires the expenditure of substantial resources. Of particular significance to us are the requirements relating to research and development and testing.
Before commencing clinical trials in humans in the United States, we, or our collaborative partners, will need to submit and receive approval from the FDA of an IND. Clinical trials are subject to oversight by institutional review boards and the FDA and:
While we have stated that we intend to file additional INDs, this is only a statement of intent, and we may not be able to do so because we may not be able to identify potential product candidates. In addition, the FDA may not approve any IND in a timely manner, or at all.
Before receiving FDA clearance to market a product, we must demonstrate that the product is safe and effective on the patient population that will be treated. Data obtained from preclinical and clinical activities are susceptible to varying interpretations that could delay, limit or prevent regulatory clearances. In addition, delays or rejections may be encountered based upon additional government regulation from future legislation or administrative action or changes in FDA policy during the period of product development, clinical trials and FDA regulatory review. Failure to comply with applicable FDA or other applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production or injunction, as well as other regulatory action against our potential products or us. Additionally, we have limited experience in conducting and managing the clinical trials necessary to obtain regulatory approval.
If regulatory clearance of a product is granted, this clearance will be limited to those disease states and conditions for which the product is demonstrated through clinical trials to be safe and efficacious. We cannot ensure that any compound developed by us, alone or with others, will prove to be safe and efficacious in clinical trials and will meet all of the applicable regulatory requirements needed to receive marketing clearance.
Outside the United States, our ability, or that of our collaborative partners, to market a product is contingent upon receiving a marketing authorization from the appropriate regulatory authorities. This foreign regulatory approval process typically includes all of the risks associated with FDA clearance described above and may also include additional risks.
If our competitors develop technologies that are more effective than ours, our commercial opportunity will be reduced or eliminated.
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. Many of the drugs that we are attempting to discover will be competing with existing therapies. In addition, a number of companies are pursuing the development of
20
pharmaceuticals that target the same diseases and conditions that we are targeting. We face competition from pharmaceutical and biotechnology companies both in the United States and abroad.
Our competitors may utilize discovery technologies and techniques or partner with collaborators in order to develop products more rapidly or successfully than we, or our collaborators, are able to do. Many of our competitors, particularly large pharmaceutical companies, have substantially greater financial, technical and human resources than we do. In addition, academic institutions, government agencies and other public and private organizations conducting research may seek patent protection with respect to potentially competitive products or technologies and may establish exclusive collaborative or licensing relationships with our competitors.
We believe that our ability to compete is dependent, in part, upon our ability to create, maintain and license scientifically-advanced technology and upon our and our collaborators' ability to develop and commercialize pharmaceutical products based on this technology, as well as our ability to attract and retain qualified personnel, obtain patent protection or otherwise develop proprietary technology or processes and secure sufficient capital resources for the expected substantial time period between technological conception and commercial sales of products based upon our technology. The failure by us or any of our collaborators in any of those areas may prevent the successful commercialization of our potential drug targets.
Our competitors might develop technologies and drugs that are more effective or less costly than any that are being developed by us or that would render our technology and potential drugs obsolete and noncompetitive. In addition, our competitors may succeed in obtaining the approval of the FDA or other regulatory agencies for product candidates more rapidly. Companies that complete clinical trials, obtain required regulatory agency approvals and commence commercial sale of their drugs before their competitors may achieve a significant competitive advantage, including certain patent and FDA marketing exclusivity rights that would delay or prevent our ability to market certain products. Any drugs resulting from our research and development efforts, or from our joint efforts with our existing or future collaborative partners, might not be able to compete successfully with competitors' existing or future products or obtain regulatory approval in the United States or elsewhere.
Our ability to generate revenues will be diminished if our collaborative partners fail to obtain acceptable prices or an adequate level of reimbursement for products from third-party payors or government agencies.
The drugs we hope to develop may be rejected by the marketplace due to many factors, including cost. Our ability to commercially exploit a drug may be limited due to the continuing efforts of government and third-party payors to contain or reduce the costs of health care through various means. For example, in some foreign markets, pricing and profitability of prescription pharmaceuticals are subject to government control. In the United States, we expect that there will continue to be a number of federal and state proposals to implement similar government control. In addition, increasing emphasis on managed care in the United States will likely continue to put pressure on the pricing of pharmaceutical products. Cost control initiatives could decrease the price that any of our collaborators would receive for any products in the future. Further, cost control initiatives could adversely affect our collaborators' ability to commercialize our products and our ability to realize royalties from this commercialization.
Our ability to commercialize pharmaceutical products with collaborators may depend, in part, on the extent to which reimbursement for the products will be available from:
21
Significant uncertainty exists as to the reimbursement status of newly-approved healthcare products. Third-party payors, including Medicare, are challenging the prices charged for medical products and services. Government and other third-party payors increasingly are attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for new drugs and by refusing, in some cases, to provide coverage for uses of approved products for disease indications for which the FDA has not granted labeling approval. Third-party insurance coverage may not be available to patients for any products we discover and develop, alone or with collaborators. If government and other third-party payors do not provide adequate coverage and reimbursement levels for our products, the market acceptance of these products may be reduced.
If product liability lawsuits are successfully brought against us, we may incur substantial liabilities and may be required to limit commercialization of our products.
The testing and marketing of medical products entail an inherent risk of product liability. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our products although we are not currently aware of any specific causes for concern with respect to clinical liability claims. We currently do not have product liability insurance, and our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of pharmaceutical products we develop, alone or with corporate collaborators. We, or our corporate collaborators, might not be able to obtain insurance at a reasonable cost, if at all. While under various circumstances we are entitled to be indemnified against losses by our corporate collaborators, indemnification may not be available or adequate should any claim arise.
Our research and development efforts will be seriously jeopardized, if we are unable to attract and retain key employees and relationships.
As a small company with only 144 employees as of December 31, 2004, our success depends on the continued contributions of our principal management and scientific personnel and on our ability to develop and maintain important relationships with leading academic institutions, scientists and companies in the face of intense competition for such personnel. In particular, our research programs depend on our ability to attract and retain highly skilled chemists, other scientists, and regulatory and clinical personnel. If we lose the services of any of our personnel, our research and development efforts could be seriously and adversely affected. Our employees can terminate their employment with us at any time.
We depend on various scientific consultants and advisors for the success and continuation of our research and development efforts.
We work extensively with various scientific consultants and advisors. The potential success of our drug discovery and development programs depends, in part, on continued collaborations with certain of these consultants and advisors. We, and various members of our management and research staff, rely on certain of these consultants and advisors for expertise in our research, regulatory and clinical efforts. Our scientific advisors are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. We do not know if we will be able to maintain such consulting agreements or that such scientific advisors will not enter into consulting arrangements, exclusive or otherwise, with competing pharmaceutical or biotechnology companies, any of which would have a detrimental impact on our research objectives and could have a material adverse effect on our business, financial condition and results of operations.
22
If we use biological and hazardous materials in a manner that causes injury or violates laws, we may be liable for damages.
Our research and development activities involve the controlled use of potentially harmful biological materials as well as hazardous materials, chemicals and various radioactive compounds. We cannot completely eliminate the risk of accidental contamination or injury from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for damages that result, and such liability could exceed our resources. We are also subject to federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. The cost of compliance with, or any potential violation of, these laws and regulations could be significant.
Our facilities are located near known earthquake fault zones, and the occurrence of an earthquake or other catastrophic disaster could cause damage to our facilities and equipment, which could require us to cease or curtail operations.
Our facilities are located in the San Francisco Bay Area near known earthquake fault zones and are vulnerable to significant damage from earthquakes. We are also vulnerable to damage from other types of disasters, including fires, floods, power loss, communications failures and similar events. If any disaster were to occur, our ability to operate our business at our facilities would be seriously, or potentially completely, impaired, and our research could be lost or destroyed. In addition, the unique nature of our research activities and of much of our equipment could make it difficult for us to recover from a disaster. The insurance we maintain may not be adequate to cover or losses resulting from disasters or other business interruptions.
Our stock price may be volatile, and our stockholders' investment in our stock could decline in value.
The market prices for our securities and those of other biotechnology companies have been highly volatile and may continue to be highly volatile in the future. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our common stock:
23
Anti-takeover provisions in our charter documents and under Delaware law may make an acquisition of us, which may be beneficial to our stockholders, more difficult.
Provisions of our amended and restated certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us, even if doing so would benefit our stockholders. These provisions:
In addition, Section 203 of the Delaware General Corporation Law, which imposes certain restrictions relating to transactions with major stockholders, may discourage, delay or prevent a third party from acquiring us.
We currently lease facilities consisting of approximately 147,000 square feet of research and office space located at 1180 Veterans Boulevard, South San Francisco, California. In May 2004, we subleased approximately 15,000 square feet of our space to a tenant for a period of two years. We believe our facilities are in good operating condition and that the leased real property is adequate for all present and near term uses.
None.
Item 4. Submission of Matters to a Vote of Security Holders
None.
24
Item 5. Market for the Registrant's Common Equity and Related Stockholder Matters
Our common stock has traded on the Nasdaq National Market under the symbol "RIGL" since November 29, 2000. The following table sets forth, for the periods indicated, the high and low sales prices for the common stock as reported by the Nasdaq National Market:
| |
High |
Low |
|||||
|---|---|---|---|---|---|---|---|
| Year Ended December 31, 2003 | |||||||
| First Quarter | $ | 11.79 | $ | 4.91 | |||
| Second Quarter | $ | 15.39 | $ | 5.31 | |||
| Third Quarter | $ | 15.00 | $ | 7.00 | |||
| Fourth Quarter | $ | 19.25 | $ | 12.08 | |||
| Year Ended December 31, 2004 | |||||||
| First Quarter | $ | 26.50 | $ | 16.20 | |||
| Second Quarter | $ | 23.66 | $ | 12.92 | |||
| Third Quarter | $ | 25.32 | $ | 10.86 | |||
| Fourth Quarter | $ | 29.00 | $ | 22.40 | |||
The sales prices in the above table reflect a one-for-nine reverse split of shares of our outstanding common stock effected on June 24, 2003. On February 28, 2005, the last reported sale price for our common stock on the Nasdaq National Market was $18.64 per share.
Holders
As of February 28, 2005, there were approximately 315 stockholders of record of our common stock.
Dividends
We have not paid dividends on our common stock and currently do not plan to pay any cash dividends in the foreseeable future.
25
Item 6. Selected Financial Data
The following selected financial data should be read in conjunction with "Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Item 8. Financial Statements and Supplementary Data" included elsewhere in this annual report on Form 10-K.
| |
Fiscal Years Ended December 31, |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2004 |
2003 |
2002 |
2001 |
2000 |
||||||||||||
| |
(in thousands, except per share amounts) |
||||||||||||||||
| Statements of Operations Data: | |||||||||||||||||
| Contract revenues from collaborations | $ | 4,733 | $ | 11,055 | $ | 15,788 | $ | 15,303 | $ | 13,218 | |||||||
| Costs and expenses: | |||||||||||||||||
| Research and development | 48,523 | 41,649 | 40,800 | 30,806 | 30,866 | ||||||||||||
| General and administrative | 13,077 | 10,233 | 12,004 | 9,457 | 7,857 | ||||||||||||
| 61,600 | 51,882 | 52,804 | 40,263 | 38,723 | |||||||||||||
| Loss from operations | (56,867 | ) | (40,827 | ) | (37,016 | ) | (24,960 | ) | (25,505 | ) | |||||||
| Loss on disposal/sale of property and equipment | (30 | ) | (169 | ) | — | — | — | ||||||||||
| Interest income | 966 | 374 | 856 | 1,957 | 1,078 | ||||||||||||
| Interest expense | (324 | ) | (575 | ) | (870 | ) | (802 | ) | (933 | ) | |||||||
| Net loss | $ | (56,255 | ) | $ | (41,197 | ) | $ | (37,030 | ) | $ | (23,805 | ) | $ | (25,360 | ) | ||
| Deemed dividend to Series E preferred stockholders | — | — | — | — | (10,133 | ) | |||||||||||
| Loss allocable to common stockholders | $ | (56,255 | ) | $ | (41,197 | ) | $ | (37,030 | ) | $ | (23,805 | ) | $ | (35,493 | ) | ||
| Loss per common share, basic and diluted | $ | (3.12 | ) | $ | (3.62 | ) | $ | (7.41 | ) | $ | (5.75 | ) | $ | (43.98 | ) | ||
| Weighted average common shares used in computing loss per common share, basic and diluted | 18,053 | 11,395 | 4,995 | 4,143 | 807 | ||||||||||||
| Pro forma loss per common share, basic and diluted | $ | (10.81 | ) | ||||||||||||||
| Shares used in computing pro forma loss per common share, basic and diluted | 3,283 | ||||||||||||||||
| |
As of December 31, |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2004 |
2003 |
2002 |
2001 |
2000 |
|||||||||||
| |
(in thousands) |
|||||||||||||||
| Balance Sheet Data: | ||||||||||||||||
| Cash, cash equivalents and available-for-sale securities | $ | 71,427 | $ | 46,500 | $ | 27,291 | $ | 33,415 | $ | 52,994 | ||||||
| Working capital | 62,821 | 41,907 | 22,493 | 26,371 | 46,627 | |||||||||||
| Total assets | 78,822 | 55,524 | 44,342 | 46,448 | 64,262 | |||||||||||
| Capital lease obligations, less current portion | 781 | 1,236 | 2,313 | 4,243 | 5,761 | |||||||||||
| Deferred stock compensation | (56 | ) | (200 | ) | (772 | ) | (2,452 | ) | (5,792 | ) | ||||||
| Accumulated deficit | (212,266 | ) | (156,011 | ) | (114,814 | ) | (77,784 | ) | (53,979 | ) | ||||||
| Total stockholders' equity | 52,301 | 39,973 | 25,441 | 28,941 | 49,010 | |||||||||||
The share numbers set forth in the table reflect a one-for-nine reverse split of shares of our outstanding common stock effected on June 24, 2003. See Notes to the Financial Statements for description of the number of shares used in the computation of basic and diluted loss per common share.
26
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
Except for the historical information contained herein, the following discussion contains forward-looking statements that are based upon current expectations. Forward-looking statements involve risks and uncertainties and include statements related to:
When used herein, the words "believe," "anticipate," "expect," "estimate," "plan" and similar expressions are intended to identify such forward-looking statements. There can be no assurance that these statements will prove to be correct. Our actual results and the timing of events could differ significantly from those discussed here. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in "Risk Factors" in Part I, as well as those discussed elsewhere in this annual report on Form 10-K. You should consider our forward-looking statements in light pf the risks discussed in "Risk Factors," as well as our financial statements, related notes, and the other financial information appearing elsewhere in this report. Rigel undertakes no obligation to update any of the forward-looking statements contained herein to reflect any future events or developments.
Overview
Rigel Pharmaceuticals, Inc. discovers and develops novel, small-molecule compounds for the treatment of large, unmet medical needs. Our objective is to create a portfolio of product candidates that can be developed for our own proprietary programs and with potential collaborative partners. Our productive discovery engine enables us to move one product candidate into the clinic each year. Currently, we have product development programs for the indications of allergy/asthma, rheumatoid arthritis, hepatitis C and cancer.
Our objective is creating a portfolio of product candidates that can be developed into small molecule therapeutics for our own proprietary programs and with potential collaborative partners. We believe that producing a portfolio of many product candidates and working in conjunction with pharmaceutical companies increases our probability of commercial success. The product development process is one that is subject to both high costs and high risk of failure. We believe that this approach allows us to minimize the risk of failure, while concurrently strategically placing us in a position to help fill the continuing product pipeline gap at major pharmaceutical companies.
Over the last couple of years, we have matured into a drug development company with multiple product candidates in candidates in various stages of development.
27
In addition to the above mentioned product candidates, we have ongoing research programs involving back-up candidates for the four product candidates above as well as drug discovery efforts in our immunology, virology, and oncology programs.
Corporate Collaborations
In addition to the preceding programs in which we retain all commercial and economic rights, we also carry on research and development programs in connection with our corporate collaborations. As of December 31, 2004, we have collaborations with five major pharmaceutical companies, including one with Janssen Pharmaceutica N.V., a division of Johnson & Johnson, relating to oncology therapeutics and diagnostics, two with Pfizer Inc., one initiated in 1999 and the other in 2005, relating to asthma and allergy therapeutics, one with Novartis Pharma AG with respect to four different programs relating to immunology, oncology and chronic bronchitis, one with Daiichi Pharmaceuticals Co., Ltd. in the area of oncology, and one with Merck, also in the area of oncology. These collaborations all have a research phase during which we receive or received funding based on the level of headcount allocated to a program. In all of these collaborations, after the research phase concludes, if certain conditions are met, we are entitled to receive future milestone payments and royalties. We cannot guarantee that these conditions will be met or that research and development efforts will be successful. As a result, we may be precluded from receiving any milestone payments or royalties under these agreements. Only the Daiichi and Merck programs are currently in the research phase of their respective agreements and provide for regular research reimbursement payments. The research phase of the Daiichi collaboration will end in August 2005.
We are exploring new opportunities with existing and potential collaborators. Our earliest partnerships focused on the early stages of drug discovery, specifically on target discovery and validation. Our collaborations with Daiichi and recently with Merck are both later stage focusing on drug discovery and development. Our 2005 collaboration with Pfizer covered compounds at the preclinical and lead designation stages. We currently anticipate that in order to support our current research programs we will need to self-fund our own research programs, which involve an increased rate of spending, to later stages of development prior to partnering with collaborative partners. Therefore, it is expected that future collaborations may have an expanded focus and could include HTS, combinatorial and medicinal chemistry, preclinical evaluations and/or clinical development of compounds we have discovered. In addition, we believe these future collaborations could be structured to consist of upfront payments, milestone payments upon meeting certain conditions, research reimbursement payments and/or royalties upon commercialization of products resulting from the collaboration.
28
Critical Accounting Policies and the Use of Estimates
Our discussion and analysis of our financial condition and results of operations are based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates, including those related to terms of the research collaborations, investments, stock compensation, impairment issues, the estimated useful life of assets and contingencies. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our financial statements.
We believe that there have been no significant changes in our critical accounting policies during the year ended December 31, 2004 as compared to those previously disclosed in our Annual Report on Form 10-K for the year ended December 31, 2003.
Revenue Recognition
We recognize revenue from our contract arrangements. Our revenue arrangements with multiple elements are divided into separate units of accounting if certain criteria are met, including whether the delivered element has stand-alone value to the customer and whether there is objective and reliable evidence of the fair value of the undelivered items. The consideration we receive is allocated among the separate units based on their respective fair values, and the applicable revenue recognition criteria are applied to each of the separate units. Advance payments received in excess of amounts earned are classified as deferred revenue until earned.
Non-refundable, up-front payments received in connection with research and development collaboration agreements, including technology access fees, are deferred and recognized on a straight-line basis over the relevant periods of continuing involvement, generally the research term.
Revenues related to collaborative research with our corporate collaborators are recognized as research services are performed over the related development funding periods for each contract. Under these agreements, we are required to perform research and development activities as specified in each respective agreement. The payments received are not refundable and are generally based on a contractual cost per full-time equivalent employee working on the project. Research and development expenses under the collaborative research agreements approximate or exceed the revenue recognized under such agreements over the term of the respective agreements. Deferred revenue may result if we were not to incur the required level of effort during a specific period in comparison to funds received under the respective contracts.
Milestones are recognized pursuant to collaborative agreements upon the achievement of these specified at risk milestones.
Royalties are expected to be recognized as earned in accordance with the contract terms when the third party results are reliably measurable and collectibility is reasonably assured.
29
Stock-based Compensation
| |
|
|
|
Aggregate Change |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Years Ended December 31, |
|||||||||||||||
| |
2004 from 2003 |
2003 from 2002 |
||||||||||||||
| |
2004 |
2003 |
2002 |
|||||||||||||
| |
(in thousands) |
|||||||||||||||
| Stock-based compensation from: | ||||||||||||||||
| Re-priced options | $ | 1,900 | $ | 1,124 | $ | — | $ | 776 | $ | 1,124 | ||||||
| Consultant options | 430 | 158 | (196 | ) | 272 | 354 | ||||||||||
| Other employee options | 236 | (167 | ) | 955 | 403 | (1,122 | ) | |||||||||
| Total | $ | 2,566 | $ | 1,115 | $ | 759 | $ | 1,451 | 356 | |||||||
Re-priced Options
We record charges associated with the stock options that were eligible for re-pricing under a tender offer initiated in June 2003. All replacement options, as well as the eligible options that were not surrendered under the original offer to exchange, are being treated for financial reporting purposes as variable awards. Therefore, we are recording a non-cash charge (recovery), generally for the intrinsic value of the options as they vest, utilizing the graded vesting method, reflecting increases and decreases (down to, but not below, the exercise price) in the price of our common stock as compensation expense (recovery) in connection with the replacement options and the eligible options that were not exchanged. We expect to continue to reflect increases and decreases in the price of our common stock in our statement of operations with respect to these options until they are exercised, forfeited or terminated. The higher the market value of our common stock, the greater the compensation expense. For the year ended December 31, 2004, we recorded a non-cash compensation charge of $1.9 million related to all options eligible for the replacement until the options are exercised, forfeited, or terminated. For the year ended December 31, 2003, we recorded a non-cash compensation charge of $1.1 million related to all options eligible for the replacement. In both years, the charge resulted from the increase in the market price of our common stock during the respective year. We are currently evaluating option valuation methodologies and assumptions in light of SFAS 123R related to our employee stock option and employee stock purchase plans.
Consultant Options
We also record charges associated with options granted to consultants reflecting the periodic revaluation of outstanding unvested consultant options based upon the current market value of our common stock and other assumptions, including the expected future volatility of our stock price. We recognized stock-based compensation for revaluation of consultant options of $430,000 and $158,000 for the years ended December 31, 2004 and 2003, respectively. We recognized stock-based compensation recovery for the revaluation of consultant options of $196,000 for the year ended December 31, 2002. We expect to see continued fluctuations in the future as a portion of these options are revalued based on the changes in the current market price of our common stock through the application of the graded vesting method.
Other employee options
We recorded $58,000 of deferred stock compensation for the year ended December 31, 2004. We recorded no deferred stock compensation with respect to options granted to employees for the years ended December 31, 2003 and 2002. The amount recorded in 2004 related to an option grant to a certain executive officer that was modified to reduce the vesting term. This amount has been reflected as a component of stockholders' equity, and the deferred expense is being amortized to operations over the remaining vesting period of the option grant using the graded vesting method. As a result of our
30
reduction in force on January 31, 2003, we recognized approximately $599,000 of stock-based compensation recovery associated with the unvested and cancelled options of the terminated employees which had previously been recognized under the graded vesting method of deferred compensation amortization. We amortized deferred stock compensation of $0.1 million, $0.6 million and $1.7 million for the years ended December 31, 2004, 2003 and 2002, respectively. At December 31, 2004, we had a total of $56,000 remaining to be amortized over the remaining vesting periods of the stock options.
Results of Operations
Years Ended December 31, 2004, 2003 and 2002
Revenues
| |
|
|
|
Aggregate Change |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Years Ended December 31, |
|||||||||||||||
| |
2004 from 2003 |
2003 from 2002 |
||||||||||||||
| |
2004 |
2003 |
2002 |
|||||||||||||
| |
(in thousands) |
|||||||||||||||
| Contract revenues from collaborations | $ | 4,733 | $ | 11,055 | $ | 15,788 | $ | (6,322 | ) | $ | (4,733 | ) | ||||
Revenues by collaborator were:
| |
|
|
|
Aggregate Change |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Years Ended December 31, |
|||||||||||||||
| |
2004 from 2003 |
2003 from 2002 |
||||||||||||||
| |
2004 |
2003 |
2002 |
|||||||||||||
| |
(in thousands) |
|||||||||||||||
| Daiichi | $ | 2,629 | $ | 4,461 | $ | 971 | $ | (1,832 | ) | $ | 3,490 | |||||
| Novartis | 1,666 | 4,119 | 11,074 | (2,453 | ) | (6,955 | ) | |||||||||
| Merck | 438 | — | — | 438 | — | |||||||||||
| Johnson & Johnson | — | 2,475 | 2,850 | (2,475 | ) | (375 | ) | |||||||||
| Pfizer | — | — | 893 | — | (893 | ) | ||||||||||
| Total | $ | 4,733 | $ | 11,055 | $ | 15,788 | $ | (6,322 | ) | $ | (4,733 | ) | ||||
Contract revenues from collaborations in 2004, 2003 and 2002 consisted primarily of research support and amortization of upfront fees from the continuation of our collaborations. Revenues for 2003 and 2002 also included milestone payments from certain collaborators. The decrease in 2004 revenues of $6.3 million was primarily due to the termination of the research phase of the Novartis oncology program and the termination of the research phase of the Johnson & Johnson oncology program. Also, revenues in 2003 included a $1.9 million milestone payment from Daiichi for the completion of a certain screening phase of the collaboration. The overall decrease in revenues in 2003 of $4.7 million was primarily due to the termination of the research phase of the Novartis T-cell and B-cell programs and the termination of the research phase of the 1999 Pfizer collaboration. The decrease in revenues in 2003 was offset by the $1.9 million milestone payment from Daiichi and a full year of revenue from the Daiichi collaboration. As of December 31, 2004, only Daiichi and Merck were in the research phase of their agreements. The research phase of the Daiichi collaboration ends in July 2005 while the research phase of the Merck collaboration ends in May 2007. In January 2005, we signed a collaborative research and license agreement with Pfizer for the development of inhaled products for the treatment of allergic asthma and other respiratory diseases focusing on our small molecule compounds that inhibit Syk kinase. We expect contract revenues from collaborations to continue to be a significant component of our total revenues for the foreseeable future.
31
Research and Development
| |
|
|
|
Aggregate Change |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Years Ended December 31, |
||||||||||||||
| |
2004 from 2003 |
2003 from 2002 |
|||||||||||||
| |
2004 |
2003 |
2002 |
||||||||||||
| |
(in thousands) |
||||||||||||||
| Research and development expenses | $ | 48,523 | $ | 41,649 | $ | 40,800 | $ | 6,874 | $ | 849 | |||||
| Stock based compensation expense included in research and development expenses | $ | 2,000 | $ | 924 | $ | 567 | $ | 1,076 | $ | 357 | |||||
The increase in research and development expenses of $6.9 million in 2004 was primarily attributable to an increase in our preclinical and clinical costs and a stock-based compensation expense charge related to the re-priced stock options subject to variable accounting, as discussed previously under "Stock-Based Compensation" in the "Critical Accounting Policies and the Use of Estimates" section. The increase in our clinical costs in 2004 was attributable to costs associated with our Phase II clinical trial for R112, our Phase I/II clinical trial for R803, and our Phase I clinical trial for R406. The increase in our pre-clinical costs in 2004 was primarily attributable to the studies performed with R406 to enable the initiation of clinical trials in December 2004. The increase in research and development expenses of $0.8 million in 2003 was primarily attributable to a substantial increase in our facility costs associated with the move to our new building in February 2003 offset by reductions in contract chemistry, lab supplies, and research headcount. The increase in 2003 was also attributable to the stock-based compensation expense charge related to the re-priced stock options subject to variable accounting.
The scope and magnitude of future research and development expenses are difficult to predict at this time given the number of studies that will need to be conducted for any of our potential products, as well as our limited capital resources. In general, biopharmaceutical-development involves a series of steps—beginning with identification of a potential target and including, among others, proof of concept in animals and Phase I, II and III clinical studies in humans—each of which is typically more expensive than the previous step. Success in development therefore results in increasing expenditures. Our research and development expenditures currently include costs for scientific personnel, supplies, equipment, consultants, sponsored research, allocated facility costs, costs related to preclinical and clinical trials, and stock-based compensation.
Because of the number of research projects we have ongoing at any one time, and the ability to utilize resources across several projects, the majority of our research and development costs are not directly tied to any individual project and are allocated among multiple projects. Our project management is based primarily on scientific data and supplemented by these cost allocations, which are based primarily on human resource time incurred on each project. As a result, the costs allocated to a project do not necessarily reflect the actual costs of the project.
General and Administrative Expenses
| |
|
|
|
Aggregate Change |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Years Ended December 31, |
|||||||||||||||
| |
2004 from 2003 |
2003 from 2002 |
||||||||||||||
| |
2004 |
2003 |
2002 |
|||||||||||||
| |
(in thousands) |
|||||||||||||||
| General and administrative expenses | $ | 13,077 | $ | 10,233 | $ | 12,004 | $ | 2,844 | $ | (1,771 | ) | |||||
| Stock based compensation expense included in general and administrative expenses | $ | 566 | $ | 191 | $ | 192 | $ | 375 | $ | (1 | ) | |||||
The increase in general and administrative expenses of $2.8 million in 2004 was primarily attributable to an increase in our intellectual property legal costs to expand our patent estate, an increase in our stock-based compensation expense charge related to the re-priced stock options subject
32
to variable accounting, as discussed previously under "stock-based compensation" in the "Critical Accounting Policies and the Use of Estimates" section, and an increase in personnel related costs associated with our 2004 bonus plan. The decrease in general and administrative expenses of $1.8 million in 2003 was primarily attributable to a decrease in our intellectual property legal costs and personnel related costs offset by an increase in our facility costs associated with the move to our new building in February 2003.
Loss on Disposal/Sale of Property and Equipment
| |
Years Ended December 31, |
Aggregate Change |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2004 from 2003 |
2003 from 2002 |
||||||||
| |
2004 |
2003 |
2002 |
|||||||
| |
(in thousands) |
|||||||||
| Loss on disposal/sale of property and equipment | $30 | $169 | $— | $(139 | ) | $169 | ||||
During 2004, we wrote-off $4,900,000 of assets at their original acquisition cost and related accumulated depreciation of $4,870,000 for assets that are no longer in use. We recorded loss on disposal of property and equipment of $30,000. In conjunction with our move to our new facilities in February 2003, we sold to the new tenant of our previous facility certain furniture and equipment that would no longer be needed at our new location. This sale resulted in cash proceeds of approximately $71,000 and a loss on sale of $169,000. The loss represents the remaining net book value of those assets net of the cash received on the sale.
Net Interest Income/(Expense)
| |
|
|
|
Aggregate Change |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Years Ended December 31, |
|||||||||
| |
2004 from 2003 |
2003 from 2002 |
||||||||
| |
2004 |
2003 |
2002 |
|||||||
| |
(in thousands) |
|||||||||
| Net interest income/(expense) | $642 | $(201) | $(14) | $843 | $(187) | |||||
Interest income results from our interest-bearing cash and investment balances, whereas interest expense is the result of our capital lease obligations associated with fixed asset acquisitions. In 2004, interest income exceeded interest expense due primarily to the increase in our cash and investment balances resulting from our follow-on offering completed early in 2004. In 2003 and 2002, interest expense exceeded interest income primarily due to a reduction in interest rates earned on investment balances.
Future Accounting Requirements
At its March 2004 meeting, the EITF reached a consensus on recognition and measurement guidance previously discussed under EITF 03-01. The consensus clarified the meaning of other-than-temporary impairment and its application to investments classified as either available-for-sale or held-to-maturity under SFAS No. 115 "Accounting for Certain Investments in Debt and Equity Securities" and to investments accounted for under the cost method or the equity method. In September 2004, the FASB delayed indefinitely the recognition and measurement guidance to be applied to other-than-temporary impairment evaluations; however the disclosure requirements remain effective and have been adopted for our year ended December 31, 2004. We will evaluate the effect, if any, of recognition and measurement provisions of EITF Issue 03-1 when final guidance is released.
In December 2004, the FASB issued Statement No. 123R, "Share-Based Payment"–an amendment of FASB Statement Nos. 123 "Accounting for Stock-Based Compensation." and 95, "Statement of Cash Flows." SFAS 123R addresses the accounting for transactions in which a company receives employee services in exchange for (a) equity instruments of the company or (b) liabilities that are based on the
33
fair value of the company's equity instruments or that may be settled by the issuance of such equity instruments. SFAS 123R eliminates accounting for share-based compensation transactions using APB 25 (Accounting for Stock Issued to Employees) and requires instead that such transactions be accounted for using a fair-value based method, thereby requiring companies to recognize an expense for compensation cost related to share-based payment arrangements, including stock options and employee stock purchase plans. This statement is required to be adopted by us beginning July 1, 2005. The cumulative effect of adoption applied on a modified prospective basis would be measured and recognized beginning July 1, 2005. We are currently evaluating option valuation methodologies and assumptions in light of SFAS 123R related to our employee stock option and employee stock purchase plans. Current estimates of option values using the Black-Scholes method reflected in Note 1 to our financial statements appearing elsewhere in this report may not be indicative of results from valuation methodologies ultimately adopted by us in compliance with SFAS 123R.
Liquidity and Capital Resources
Cash Requirements
We have financed our operations from inception primarily through sales of equity securities, contract payments payable to us under our collaboration agreements and equipment financing arrangements. We believe that our existing capital resources and anticipated proceeds from current collaborations will be sufficient to support our current operating plan through at least the next twelve months. Our operations will require significant additional funding in large part due to our research and development expenses, future preclinical and clinical-testing costs, our facility lease commitments and the absence of any meaningful revenues for the foreseeable future. The amount of future funds needed will depend largely on the timing and structure of potential future collaborations. We do not know whether additional financing will be available when needed, or that, if available, we will obtain financing on terms favorable to our stockholders or us. We have consumed substantial amounts of capital to date, and operating expenditures are expected to increase over the next several years as we expand our research and development activities.
On October 15, 2004, we filed a shelf registration statement on Form S-3 with the Securities and Exchange Commission for the proposed offering, from time to time, of up to $150.0 million of our common stock, preferred stock, debt securities and/or warrants.
To the extent we raise additional capital by issuing equity securities, our stockholders would at that time experience substantial dilution. To the extent that we raise additional funds through collaboration and licensing arrangements, we may be required to relinquish some rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us. Our future funding requirements will depend upon many factors, including, but not limited to:
34
Insufficient funds may require us to delay, scale back or eliminate some or all of our research or development programs, to lose rights under existing licenses or to relinquish greater or all rights to product candidates at an earlier stage of development or on less favorable terms than we would otherwise choose or may adversely affect our ability to operate as a going concern.
As of December 31, 2004, we had $71.4 million in cash, cash equivalents and available-for-sale securities, as compared to $46.5 million as of December 31, 2003, an increase of $24.9 million. The increase was primarily attributable to net proceeds of $58.3 million, after deducting offering costs, from the follow-on offering completed in February and March 2004 in which we sold 3,135,075 shares of our common stock at a price to the public of $20.00 per share. These financing proceeds were offset by approximately $38.2 million in net cash used in operating activities. We also made debt service payments of $2.2 million in conjunction with our equipment financing arrangements. For the year ended December 31, 2004 and 2003, we maintained an investment portfolio primarily in money market funds, federal agency securities, and corporate bonds and notes. Cash in excess of immediate requirements is invested with regard to liquidity and capital preservation. Wherever possible, we seek to minimize the potential effects of concentration and degrees of risk.
Contractual Obligations
The following are our contractual commitments (by fiscal year) as of December 31, 2004 associated with debt obligations, contracted research obligations, and lease obligations:
| |
Total |
Less than 1 year |
1-3 years |
3-5 years |
More than 5 years |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(in thousands) |
||||||||||||||
| |
(in thousands) |
||||||||||||||
| Debt obligations (1) | $ | 2,286 | $ | 1,452 | $ | 834 | $ | — | $ | — | |||||
| Contracted research | 220 | 220 | — | — | — | ||||||||||
| Facilities leases, net of sublease (2)(3) | 181,635 | 12,354 | 26,307 | 28,344 | 114,630 | ||||||||||
| Total | $ | 184,141 | $ | 14,026 | $ | 27,141 | $ | 28,344 | $ | 114,630 | |||||
35
As of December 31, 2004, we had federal net operating loss carryforwards of approximately $175.9 million to offset future taxable income. We also had federal research and development tax credit carryforwards of approximately $11.2 million. If not utilized, net operating loss and credit carryforwards will begin to expire in 2011. Utilization of the net operating losses and credits may be subject to a substantial annual limitation due to ownership change limitations provided by the Internal Revenue Code of 1986. The annual limitation may result in the expiration of our net operating losses and credits before they can be used. You should read Note 8 of the notes to our financial statements.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
The primary objective of our investment activities is to preserve principal while at the same time maximizing the income we receive from our investments without significantly increasing risk. Some of the securities in which we invest may have market risk. This means that a change in prevailing interest rates may cause the fair value amount of the investment to fluctuate. For example, if we hold a security that was issued with a fixed interest rate at the then-prevailing rate and the prevailing interest rate later rises, the market value amount of our investment will decline. To minimize this risk in the future, we intend to maintain our portfolio of cash equivalents and available-for-sale securities in a variety of securities, including money market funds and government and non-government debt securities. In 2004, 2003 and 2002, we maintained an investment portfolio primarily in money market funds, federal agency securities, and corporate bonds and notes. Due to the primarily short-term nature of these investments, we believe we do not have a material exposure to interest rate risk arising from our investments. Therefore, no quantitative tabular disclosure is provided.
We have operated primarily in the United States, and all funding activities with our collaborators to date have been made in U.S. dollars. Accordingly, we have not had any exposure to foreign currency rate fluctuations.
36
Item 8. Financial Statements and Supplementary Data
INDEX TO FINANCIAL STATEMENTS
Rigel Pharmaceuticals, Inc.
| |
Page |
|
|---|---|---|
| Report of Independent Registered Public Accounting Firm on Financial Statements | 38 | |
Balance Sheets |
39 |
|
Statements of Operations |
40 |
|
Statement of Stockholders' Equity |
41 |
|
Statements of Cash Flows |
42 |
|
Notes to Financial Statements |
43 |
37
Report of Independent Registered Public Accounting Firm on Financial Statements
The
Board of Directors and Stockholders
Rigel Pharmaceuticals, Inc.
We have audited the accompanying balance sheets of Rigel Pharmaceuticals, Inc. as of December 31, 2004 and 2003, and the related statements operations, stockholders' equity, and cash flows for each of the three years in the period ended December 31, 2004. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Rigel Pharmaceuticals, Inc. at December 31, 2004 and 2003, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2004, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of Rigel Pharmaceuticals, Inc.'s internal control over financial reporting as of December 31, 2004, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 10, 2005 expressed an unqualified opinion thereon.
| /s/ ERNST & YOUNG, LLP |
Palo
Alto, California
March 10, 2005
38
RIGEL PHARMACEUTICALS, INC.
BALANCE SHEETS
(In thousands, except share and per share amounts)
| |
December 31, |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
2004 |
2003 |
||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 10,495 | $ | 9,621 | ||||
| Available-for-sale securities | 60,932 | 36,879 | ||||||
| Accounts receivable | — | 500 | ||||||
| Other receivables | 699 | 787 | ||||||
| Prepaid expenses and other current assets | 2,113 | 2,174 | ||||||
| Total current assets | 74,239 | 49,961 | ||||||
| Property and equipment, net | 2,813 | 3,544 | ||||||
| Other assets | 1,770 | 2,019 | ||||||
| $ | 78,822 | $ | 55,524 | |||||
| Liabilities and stockholders' equity | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 1,945 | $ | 1,378 | ||||
| Accrued compensation | 1,639 | 711 | ||||||
| Other accrued liabilities | 1,555 | 1,464 | ||||||
| Deferred revenue | 3,728 | 2,242 | ||||||
| Deferred rent | 1,230 | — | ||||||
| Capital lease obligations | 1,321 | 2,259 | ||||||
| Total current liabilities | 11,418 | 8,054 | ||||||
| Long-term portion of capital lease obligations | 781 | 1,236 | ||||||
| Long-term portion of deferred revenue | 4,180 | 546 | ||||||
| Long-term portion of deferred rent | 9,685 | 5,297 | ||||||
| Other long-term liabilities | 457 | 418 | ||||||
| Commitments | ||||||||
| Stockholders' equity: | ||||||||
| Common stock, $0.001 par value; 100,000,000 shares authorized; 19,661,295 and 14,828,546 shares issued and outstanding in 2004 and 2003, respectively | 20 | 15 | ||||||
| Additional paid-in capital | 264,823 | 196,215 | ||||||
| Deferred stock compensation | (56 | ) | (200 | ) | ||||
| Accumulated other comprehensive loss | (220 | ) | (13 | ) | ||||
| Accumulated deficit | (212,266 | ) | (156,011 | ) | ||||
| 52,301 | 40,006 | |||||||
Less treasury stock, at cost: none and 4,525 shares in 2004 and 2003, respectively |
— |
(33 |
) |
|||||
| Total stockholders' equity | 52,301 | 39,973 | ||||||
| $ | 78,822 | $ | 55,524 | |||||
See accompanying notes.
39
RIGEL PHARMACEUTICALS, INC.
STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| |
Years ended December 31, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2004 |
2003 |
2002 |
||||||||
| Contract revenues from collaborations | $ | 4,733 | $ | 11,055 | $ | 15,788 | |||||
| Costs and expenses: | |||||||||||
| Research and development | 48,523 | 41,649 | 40,800 | ||||||||
| General and administrative | 13,077 | 10,233 | 12,004 | ||||||||
| 61,600 | 51,882 | 52,804 | |||||||||
| Loss from operations | (56,867 | ) | (40,827 | ) | (37,016 | ) | |||||
| Loss on disposal/sale of property and equipment | (30 | ) | (169 | ) | — | ||||||
| Interest income | 966 | 374 | 856 | ||||||||
| Interest expense | (324 | ) | (575 | ) | (870 | ) | |||||
| Net loss | (56,255 | ) | (41,197 | ) | (37,030 | ) | |||||
| Net loss per common share, basic and diluted | $ | (3.12 | ) | $ | (3.62 | ) | $ | (7.41 | ) | ||
| Weighted average shares used in computing net loss per common share, basic and diluted | 18,053 | 11,395 | 4,995 | ||||||||
See accompanying notes.
40
RIGEL PHARMACEUTICALS, INC.
STATEMENT OF STOCKHOLDERS' EQUITY
(In thousands, except per share and per share amounts)
| |
Common Stock |
|
|
Accumulated Other Comprehensive Income (loss) |
|
|
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Additional Paid-in Capital |
Deferred Stock Compensation |
Accumulated Deficit |
Treasury Stock |
Total Stockholders' Equity |
||||||||||||||||||||
| |
Shares |
Amount |
|||||||||||||||||||||||
| Balance at December 31, 2001 | 4,192,467 | $ | 4 | $ | 109,129 | $ | (2,452 | ) | $ | 44 | $ | (77,784 | ) | — | $ | 28,941 | |||||||||
| Net loss | — | — | — | — | — | (37,030 | ) | — | (37,030 | ) | |||||||||||||||
| Change in unrealized gain on available-for-sale securities | — | — | — | — | (45 | ) | — | — | (45 | ) | |||||||||||||||
| Comprehensive loss | (37,075 | ) | |||||||||||||||||||||||
Issuance of common stock at $40.50 per share for cash, net of issuance costs |
777,778 |
1 |
29,427 |
— |
— |
— |
— |
29,428 |
|||||||||||||||||
| Issuance of common stock at $38.70 per share for cash, net of issuance costs | 51,678 | — | 1,923 | — | — | — | — | 1,923 | |||||||||||||||||
| Issuance of common stock upon exercise of options and participation in Purchase Plan | 56,101 | — | 446 | — | — | — | — | 446 | |||||||||||||||||
| Issuance of warrants to purchase common stock for services | — | — | 1,018 | — | — | — | — | 1,018 | |||||||||||||||||
| Compensation recovery related to options granted to consultants | — | — | (196 | ) | — | — | — | — | (196 | ) | |||||||||||||||
| Amortization of deferred stock compensation, net of cancellations | — | — | (724 | ) | 1,680 | — | — | — | 956 | ||||||||||||||||
| Balance at December 31, 2002 | 5,078,024 | 5 | 141,023 | (772 | ) | (1 | ) | (114,814 | ) | — | 25,441 | ||||||||||||||
| Net loss | — | — | — | — | — | (41,197 | ) | — | (41,197 | ) | |||||||||||||||
| Change in unrealized loss on available-for-sale securities | — | — | — | — | (12 | ) | — | — | (12 | ) | |||||||||||||||
Comprehensive loss |
(41,209 |
) |
|||||||||||||||||||||||
Issuance of common stock at $5.76 per share for cash, net of issuance costs |
7,986,110 |
8 |
34,073 |
— |
— |
— |
— |
34,081 |
|||||||||||||||||
| Issuance of common stock at $5.76 per share for cash, net of issuance costs | 1,615,705 | 2 | 9,103 | — | — | — | — | 9,105 | |||||||||||||||||
| Issuance of warrants to purchases common stock at $5.76 per share | — | — | 10,957 | — | — | — | — | 10,957 | |||||||||||||||||
| Fractional shares adjustment upon reverse split | (101 | ) | — | (1 | ) | — | — | — | — | (1 | ) | ||||||||||||||
| Issuance of common stock upon exercise of options and participation in Purchase Plan | 148,808 | — | 514 | — | — | — | — | 514 | |||||||||||||||||
| Purchase of common stock upon net exercise | — | — | — | — | — | — | (37 | ) | (37 | ) | |||||||||||||||
| Grant of treasury stock to an employee | — | — | 2 | — | — | — | 4 | 6 | |||||||||||||||||
| Compensation expense related to options granted to consultants | — | — | 159 | — | — | — | — | 159 | |||||||||||||||||
| Compensation expense related to repriced options | — | — | 1,125 | — | — | — | — | 1,125 | |||||||||||||||||
| Amortization of deferred stock compensation, net of cancellations | — | — | (740 | ) | 572 | — | — | — | (168 | ) | |||||||||||||||
| Balance at December 31, 2003 | 14,828,546 | 15 | 196,215 | (200 | ) | (13 | ) | (156,011 | ) | (33 | ) | 39,973 | |||||||||||||
| Net loss | — | — | — | — | — | (56,255 | ) | — | (56,255 | ) | |||||||||||||||
| Change in unrealized loss on available-for-sale securities | — | — | — | — | (207 | ) | — | — | (207 | ) | |||||||||||||||
Comprehensive loss |
(56,462 |
) |
|||||||||||||||||||||||
Issuance of common stock at $20.00 per share for cash, net of issuance costs |
3,135,075 |
3 |
58,338 |
— |
— |
— |
— |
58,341 |
|||||||||||||||||
| Issuance of common stock upon exercise of options and participation in Purchase Plan | 227,892 | — | 1,884 | — | — | — | — | 1,884 | |||||||||||||||||
| Issuance of common stock upon cash exercise of warrants at $5.76 | 1,041,666 | 1 | 6,000 | — | — | — | — | 6,001 | |||||||||||||||||
| Issuance of common stock upon net exercise of warrants at $5.76 | 415,687 | 1 | — | — | — | — | — | 1 | |||||||||||||||||
| Issuance of common stock upon net exercise of warrants | 12,429 | — | — | — | — | — | — | — | |||||||||||||||||
| Grant of treasury stock to employees | — | — | 39 | — | — | — | 33 | 72 | |||||||||||||||||
| Compensation expense related to options granted to consultants, repriced options, and an option modification | — | — | 2,289 | 75 | — | — | — | 2,364 | |||||||||||||||||
| Deferred stock compensation related to option modification | — | — | 58 | (58 | ) | — | — | — | — | ||||||||||||||||
| Amortization of deferred stock compensation, | — | — | — | 127 | — | — | — | 127 | |||||||||||||||||
| Balance at December 31, 2004 | 19,661,295 | $ | 20 | $ | 264,823 | $ | (56 | ) | $ | (220 | ) | $ | (212,266 | ) | $ | — | $ | 52,301 | |||||||
See accompanying notes
41
RIGEL PHARMACEUTICALS, INC.
STATEMENTS OF CASH FLOWS
(In thousands)
| |
Years ended December 31, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2004 |
2003 |
2002 |
|||||||||
| Operating activities | ||||||||||||
| Net loss | $ | (56,255 | ) | $ | (41,197 | ) | $ | (37,030 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation and amortization | 2,246 | 2,664 | 4,868 | |||||||||
| Amortization of deferred stock compensation, net | 127 | 513 | 956 | |||||||||
| Non-cash stock compensation (recovery) | 2,367 | 602 | (196 | ) | ||||||||
| Issuances of equity instruments for noncash benefits | 72 | 6 | — | |||||||||
| Loss on disposal/sale of property and equipment | 30 | 169 | — | |||||||||
| Changes in assets and liabilities: | ||||||||||||
| Accounts receivable | 500 | 869 | (556 | ) | ||||||||
| Other receivables | 88 | (520 | ) | 206 | ||||||||
| Prepaid expenses and other current assets | 61 | 5,610 | (4,593 | ) | ||||||||
| Other assets | 249 | 406 | 221 | |||||||||
| Accounts payable | 567 | (2,082 | ) | 1,480 | ||||||||
| Accrued compensation | 928 | (88 | ) | 128 | ||||||||
| Other accrued liabilities | 91 | (1,198 | ) | 74 | ||||||||
| Deferred revenue | 5,120 | (3,420 | ) | 704 | ||||||||
| Deferred rent and other long-term liabilities | 5,657 | 5,226 | (790 | ) | ||||||||
| Net cash used in operating activities | (38,152 | ) | (32,440 | ) | (34,528 | ) | ||||||
| Investing activities | ||||||||||||
| Purchases of available-for-sale securities | (96,436 | ) | (42,135 | ) | (26,713 | ) | ||||||
| Maturities of available-for-sale securities | 72,176 | 6,000 | 22,875 | |||||||||
| Sales of available-for-sale securities | — | — | 24,964 | |||||||||
| Proceeds from the sale of property and equipment | — | 71 | — | |||||||||
| Capital expenditures | (1,545 | ) | (1,242 | ) | (1,635 | ) | ||||||
| Net cash (used in) provided by in investing activities | (25,805 | ) | (37,306 | ) | 19,491 | |||||||
| Financing activities | ||||||||||||
| Proceeds from capital lease financing | 831 | 1,351 | 1,999 | |||||||||
| Payments on capital lease obligations | (2,224 | ) | (3,557 | ) | (3,712 | ) | ||||||
| Net proceeds from issuances of common stock and warrants | 66,224 | 54,620 | 31,797 | |||||||||
| Advance from landlord | — | 418 | — | |||||||||
| Net cash provided by (used in) financing activities | 64,831 | 52,832 | 30,084 | |||||||||
| Net increase (decrease) in cash and cash equivalents | 874 | (16,914 | ) | 15,047 | ||||||||
| Cash and cash equivalents at beginning of period | 9,621 | 26,535 | 11,488 | |||||||||
| Cash and cash equivalents at end of period | $ | 10,495 | $ | 9,621 | $ | 26,535 | ||||||
| Supplemental disclosure of cash flow information | ||||||||||||
| Interest paid | $ | 324 | $ | 575 | $ | 870 | ||||||
| Schedule of non cash transactions | ||||||||||||
| Deferred stock compensation | $ | 58 | — | — | ||||||||
| Issuance of warrants for services | — | — | $ | 1,018 | ||||||||
See accompanying notes.
42
Rigel Pharmaceuticals, Inc.
NOTES TO FINANCIAL STATEMENTS
In this annual report on Form 10-K, "Rigel," "we," "us" and "our" refer to Rigel Pharmaceuticals, Inc. "common stock" refers to Rigel's common stock, par value $0.001 per share.
1. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Nature of operations and basis of presentation
We were incorporated in the state of Delaware on June 14, 1996. We are engaged in the discovery and development of a broad range of new small molecule product candidates. On June 24, 2003, we effected a one-for-nine reverse stock split of our outstanding common stock, after our stockholders approved the proposal for a reverse split at our annual meeting of stockholders held on June 20, 2003. As a result of the reverse stock split, each outstanding share of common stock automatically converted into one-ninth of a share of common stock, with the par value of each share of common stock remaining at one tenth of one cent ($0.001) per share. Accordingly, common stock share and per share amounts for all periods presented have been adjusted to reflect the impact of the reverse stock split.
Financial Statement Preparation
The preparation of financial statements in conformity with U.S generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from these estimates. Certain prior year amounts have been reclassified to conform to the current year presentation.
Stock Award Plans
We have elected to continue to follow Accounting Principles Board Opinion No. 25, or APB 25, "Accounting for Stock Issued to Employees," to account for employee stock options because the alternative fair value method of accounting prescribed by Statement of Financial Accounting Standards, or FAS, No. 123, as amended by FAS No. 148 "Accounting for Stock-Based Compensation," requires the use of option valuation models that were not developed for use in valuing employee stock options. Under APB 25, the intrinsic value method of accounting, no compensation expense is recognized because the exercise price of our employee stock options equals the market price of the underlying stock on the date of grant. See "Recent Accounting Pronouncements" below for a discussion of a recently issued FASB Statement related to stock-based compensation.
Pro forma information regarding net loss and net loss per share has been determined as if we had accounted for our employee stock options and employee stock purchase plan under the fair value method prescribed by FAS 123, as amended by FAS 148. The fair value for these options was estimated at the date of grant using the Black-Scholes model with the following weighted-average assumptions for the years ended December 31, 2004, 2003 and 2002: risk-free interest rates of 2.9%, 1.3% and 2.1%, respectively; volatility of 0.85, 1.00 and 0.85 respectively; an expected option life of five years; and no dividend yield.
43
For purposes of pro forma disclosures, the estimated fair value of the options is amortized to expense over the vesting period of the options. Our pro forma information follows (in thousands, except per share amounts):
| |
Years Ended December 31, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2004 |
2003 |
2002 |
||||||||
| Net loss—as reported: | $ | (56,255 | ) | $ | (41,197 | ) | $ | (37,030 | ) | ||
| Less: Total stock-based employee compensation determined under APB 25 | 2,135 | 957 | 955 | ||||||||
| Add: Total stock-based employee compensation expense determined under the fair value based method for all awards | 7,332 | 3,066 | 3,391 | ||||||||
| Pro forma net loss | $ | (61,452 | ) | $ | (43,306 | ) | $ | (39,466 | ) | ||
| Basic and diluted net loss per common share: | |||||||||||
| As reported | $ | (3.12 | ) | $ | (3.62 | ) | $ | (7.41 | ) | ||
| Pro forma | (3.40 | ) | (3.80 | ) | (7.90 | ) | |||||
Cash, cash equivalents and available-for-sale securities
We consider all highly liquid investments in debt securities with a remaining maturity from the date of purchase of 90 days or less to be cash equivalents. Cash equivalents consist of money market funds and corporate debt securities. Our short-term investments include obligations of governmental agencies and corporate debt securities. By policy, we limit the concentration of credit risk by diversifying our investments among a variety of high credit-quality issuers.
All cash equivalents and short-term investments are classified as available-for-sale. Available-for-sale securities are carried at fair value which approximates amortized cost at December 31, 2004 and 2003. Unrealized gains (losses) are reported in stockholders' equity and included in other comprehensive income (loss). Fair value is estimated based on available market information. The cost of securities sold is based on the specific identification method. For the years ended December 31, 2004, 2003 and 2002, gross realized gains and losses on available-for-sale securities were not material. See Note 4 for a summary of available-for-sale securities at December 31, 2004 and 2003.
Fair value of financial instruments
Financial instruments, including cash and cash equivalents, accounts receivable, accounts payable, accrued liabilities and accrued compensation are carried at cost or amortized cost, which management believes approximates fair value.
Derivative financial instruments and hedging activities
All derivatives are required to be recognized on the balance sheet at fair value. Derivatives that are not designated as hedges must be adjusted to fair value through earnings. If the derivative is designated and qualifies as a hedge, depending on the nature of the hedge, changes in the fair value of the derivative are either offset against the change in fair value of assets, liabilities, or firm commitments through earnings or recognized in other comprehensive income until the hedged item is
44
recognized in earnings. The ineffective portion of a derivative's change in fair value will be immediately recognized in earnings. We do not hold derivative financial instruments and do no currently engage in hedging activities.
Property and equipment
Property and equipment are stated at cost. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets, which range from three to seven years. Leasehold improvements are amortized using the straight-line method over the estimated useful lives of the assets or the term of the lease, whichever is shorter.
Revenue recognition
We recognize revenue from our contract arrangements. Our revenue arrangements with multiple elements are divided into separate units of accounting if certain criteria are met, including whether the delivered element has stand-alone value to the customer and whether there is objective and reliable evidence of the fair value of the undelivered items. The consideration we receive is allocated among the separate units based on their respective fair values, and the applicable revenue recognition criteria are applied to each of the separate units. Advance payments received in excess of amounts earned are classified as deferred revenue until earned.
Non-refundable, up-front payments received in connection with research and development collaboration agreements, including technology access fees, are deferred and recognized on a straight-line basis over the relevant periods of continuing involvement, generally the research term.
Revenues related to collaborative research with our corporate collaborators are recognized as research services are performed over the related development funding periods for each contract. Under these agreements, we are required to perform research and development activities as specified in each respective agreement. The payments received are not refundable and are generally based on a contractual cost per full-time equivalent employee working on the project. Research and development expenses under the collaborative research agreements approximate or exceed the revenue recognized under such agreements over the term of the respective agreements. Deferred revenue may result if we were not to incur the required level of effort during a specific period in comparison to funds received under the respective contracts.
Milestones are recognized pursuant to collaborative agreements upon the achievement of these specified at risk milestones.
Royalties are expected to be recognized as earned in accordance with the contract terms when the third party results are reliably measurable and collectibility is reasonably assured.
Research and development
Research and development expenses include costs for scientific personnel, supplies, equipment, consultants, research sponsored by us, allocated facility costs, costs related to pre-clinical and clinical trials, and stock-based compensation expense. All such costs are charged to research and development expense as incurred. Collaboration agreements generally specify minimum levels of research effort required to be performed by us.
45
Impairment of long-lived assets
Long-lived assets and certain identifiable intangible assets to be held and used are reviewed for impairment when events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable.
Segment reporting
We have determined that we operate in only one segment.
Contingencies
We are subject to claims related to the patent protection of certain of our technologies. We are required to assess the likelihood of any adverse judgments or outcomes to these matters as well as potential ranges of probable losses. A determination of the amount of reserves required, if any, for these contingencies is made after careful analysis of each individual issue. The required reserves may change in the future due to new developments in each matter or changes in approach such as a change in settlement strategy in dealing with these matters.
Net loss per share
Net loss per share has been computed according to Financial Accounting Standards Board Statement No. 128, "Earnings Per Share," which requires disclosure of basic and diluted earnings per share. Basic earnings per share excludes any dilutive effects of options, shares subject to repurchase, warrants and convertible securities. Diluted earnings per share includes the impact of potentially dilutive securities.
During all periods presented, we had securities outstanding which could potentially dilute basic earnings per share in the future, but were excluded from the computation of diluted net loss per share, as their effect would have been antidilutive. These outstanding securities consist of the following (in thousands, except per share information):
| |
December 31, |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
2004 |
2003 |
2002 |
||||||
| Outstanding options | 2,750 | 2,081 | 718 | ||||||
| Warrants | 92 | 1,725 | 128 | ||||||
| Weighted average exercise price of options | $ | 11.94 | $ | 8.31 | $ | 31.23 | |||
| Weighted average exercise price of warrants | $ | 28.33 | $ | 7.17 | $ | 24.84 | |||
Recent accounting pronouncements
At its March 2004 meeting, the EITF reached a consensus on recognition and measurement guidance previously discussed under EITF 03-01. The consensus clarified the meaning of other-than-temporary impairment and its application to investments classified as either available-for-sale or held-to-maturity under SFAS No. 115 "Accounting for Certain Investments in Debt and Equity Securities" and to investments accounted for under the cost method or the equity method. In September 2004, the FASB delayed indefintely the recognition and measurement guidance to be applied to other-than-temporary impairment evaluations; however the disclosure requirements remain
46
effective and have been adopted for our year ended December 31, 2004. We will evaluate the effect, if any, of the recognition and measurement provision of EITF Issue 03-1 when final guidance is released.
In December 2004, the FASB issued Statement No. 123R, "Share-Based Payment"—an amendment of FASB Statement Nos. 123 "Accounting for Stock-Based Compensation." and 95, "Statement of Cash Flows." SFAS 123R addresses the accounting for transactions in which a company receives employee services in exchange for (a) equity instruments of the company or (b) liabilities that are based on the fair value of the company's equity instruments or that may be settled by the issuance of such equity instruments. SFAS 123R eliminates accounting for share-based compensation transactions using APB 25 (Accounting for Stock Issued to Employees) and requires instead that such transactions be accounted for using a fair-value based method, thereby requiring companies to recognize an expense for compensation cost related to share-based payment arrangements, including stock options and employee stock purchase plans. This statement is required to be adopted by us beginning July 1, 2005. The cumulative effect of adoption applied on a modified prospective basis would be measured and recognized beginning July 1, 2005. We are currently evaluating option valuation methodologies and assumptions in light of SFAS 123R related to our employee stock option and employee stock purchase plans. Current estimates of option values using the Black-Scholes method reflected in Note 1 above may not be indicative of results from valuation methodologies ultimately adopted by us in compliance with SFAS 123R.
2. SPONSORED RESEARCH AND LICENSE AGREEMENTS
Research agreements
In December 1998, we entered into a research collaboration agreement with Janssen Pharmaceutica N.V., a division of Johnson and Johnson Pharmaceutical and Development, LLC to research and identify novel targets for drug discovery. At the time we entered into the contract, Johnson & Johnson paid a one-time non-refundable, non-creditable fee and provided support for research activities during the research period, which concluded in December 2003. In addition to these fees, we also received various milestones payments. Johnson & Johnson remains obligated to pay us various milestones and royalties in the future if certain conditions are met.
In January 1999, we entered into a two-year collaborative research agreement with Pfizer Inc. to discover and develop various molecular targets. Upon signing of the agreement, Pfizer paid a one-time, nonrefundable, noncreditable fee. Under the terms of the contract, Pfizer provided support for research for two years. In 2001, Pfizer notified us that it was electing to exercise its option to extend the funded research portion of the collaboration one additional year to January 31, 2002 and then extended it again for one additional month to February 28, 2002. In February 2002, the research phase of our collaboration with Pfizer concluded with Pfizer accepting a total of seven validated targets. In January 2005, we signed a second collaborative research and license agreement with Pfizer for the development of inhaled products for the treatment of allergic asthma and other respiratory diseases focusing on our small molecule compounds that inhibit Syk kinase. Pursuant to the terms of this collaboration, we received an upfront cash payment, and are eligible to receive milestone payments and royalties on any future product sales. Pfizer made an equity investment in Rigel at a premium and will be responsible for the worldwide development and commercialization of any resulting products.
In May 1999, we entered into a broad collaboration with Novartis Pharma AG, pursuant to which we and Novartis agreed to work on up to five different research programs to identify various targets for
47
drug development. Two programs were initiated in 1999 while the third program to be conducted at Novartis was initiated on January 1, 2000. In July 2001, we expanded our collaboration with Novartis with the initiation of our angiogenesis program, the fourth and final program in our Novartis collaboration as Novartis chose not to exercise its option to add a fifth project that was to be conducted at Novartis. Pursuant to the expanded Novartis collaboration, we received a $4.0 million up-front payment from Novartis, which was recognized as revenue ratably through July 2004. We currently have no programs with Novartis in the research phase, however Novartis remains obligated to provide payment for various milestones and royalties in the future if certain conditions in the collaboration agreement are met.
In August 2002, we signed an agreement for the establishment of a collaboration with Daiichi to pursue research related to a specific target from a novel class of drug targets called ligases that control cancer cell proliferation through protein degradation. Per the agreement, the research phase of this three-year collaboration is set to expire in August 2005. Under the terms of the collaboration agreement, Daiichi paid us $0.9 million at the time we entered into the agreement, two milestone payments totaling $3.7 million, is obligated to pay us ongoing research support through July 2005 and may become obligated to pay us certain other milestones payments in the future. In addition, we are entitled to receive royalties on any commercialized products to emerge from the collaboration. Under terms of the agreement, we retain the rights to co-develop and co-promote products resulting from this collaboration in North America while Daiichi retains co-development and promotion rights in the remainder of the world.
In November 2004, we entered into a broad collaboration agreement with Merck & Co., Inc. to investigate ubiquitin ligases, a new class of drug target, to find treatments for cancer and potentially other diseases. At the time we entered into the agreement, we received an initial cash payment of $7.6M and funding for our research scientists for two and a half years. We are recognizing the upfront payment ratably over the two and a half year term of the research agreement. We are also eligible to receive milestone payments for preclinical and clinical events in the future. Merck is responsible for worldwide development and commercialization of any resulting compounds and will pay Rigel royalties on future product sales, if any. The collaboration is based on a number of new targets designated by Merck and do not include our current ligase targets. In addition, we may nominate our own targets for potential inclusion in the collaboration.
48
3. SIGNIFICANT CONCENTRATIONS
For the year ended December 31, 2004, Daiichi, Novartis and Merck accounted for 56%, 35% and 9% of total revenues, respectively. For the year ended December 31, 2003, Daiichi, Novartis and Johnson and Johnson accounted for 41%, 37% and 22% of total revenues, respectively. For the year ended December 31, 2002, Novartis, Johnson and Johnson, Daiichi and Pfizer accounted for 70%, 18%, 6% and 6% of total revenues, respectively. Rigel does not require collateral or other security for accounts receivable.
4. CASH, CASH EQUIVALENTS, AND AVAILABLE-FOR-SALE SECURITIES
Available-for-sale securities consist of the following (in thousands):
| |
Amortized Cost and Fair Value at December 31, |
|||||
|---|---|---|---|---|---|---|
| |
2004 |
2003 |
||||
| Checking account | $ | 240 | $ | 589 | ||
| Money market funds | 9,261 | 6,527 | ||||
| Federal agency securities | 15,684 | 5,996 | ||||
| Corporate bonds and notes | 46,242 | 33,388 | ||||
| $ | 71,427 | $ | 46,500 | |||
| Reported as: | ||||||
| Cash and cash equivalents | $ | 10,495 | $ | 9,621 | ||
| Available-for sale-securities | 60,932 | 36,879 | ||||
| $ | 71,427 | $ | 46,500 | |||
| |
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2004 | |||||||||||||
| Federal agency securities | $ | 15,709 | $ | 2 | $ | (27 | ) | $ | 15,684 | ||||
| Corporate bonds and note | 46,437 | 6 | (201 | ) | 46,242 | ||||||||
| Total | $ | 62,146 | $ | 8 | $ | (228 | ) | $ | 61,926 | ||||
| |
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2003 | |||||||||||||
| Federal agency securities | $ | 5,995 | $ | 1 | — | $ | 5,996 | ||||||
| Corporate bonds and note | $ | 33,402 | 2 | (16 | ) | 33,388 | |||||||
| Total | $ | 39,397 | $ | 3 | $ | (16 | ) | $ | 39,384 | ||||
49
As of December 31, 2004, the contractual maturities of debt securities were (in thousands):
| |
Years to Maturity |
|||||
|---|---|---|---|---|---|---|
| |
Within One year |
After One year through Five Years |
||||
| Federal agency securities | $ | 15,684 | $ | — | ||
| Corporate bonds and notes | 45,207 | 1,035 | ||||
| Total | $ | 60,891 | $ | 1,035 | ||
At December 31, 2004, the above debt securities had a weighted average maturity of approximately 135 days. There were no material gross realized gains or losses from sales of securities in the periods presented. We intend to hold all investments as of December 31, 2004 to maturity.
The following table shows the gross unrealized losses and fair values of our investments in individual securities that have been in a continuous unrealized loss position deemed to be temporary for less than twelve months aggregated by investment category, (in thousands):
| |
Fair Value |
Unrealized (Losses)/gains |
||||||
|---|---|---|---|---|---|---|---|---|
| 2004 | ||||||||
| Federal agency securities | $ | 11,706 | $ | (27 | ) | |||
| Corporate bonds and notes | 37,294 | (201 | ) | |||||
| Total | $ | 49,000 | $ | (228 | ) | |||
| 2003 | ||||||||
| Corporate bonds and notes | $ | 25,974 | (16 | ) | ||||
At December 31, 2004, we did not have any investments in individual securities that have been in a continuous unrealized loss position deemed to be other than temporary for more than twelve months. As of December 31, 2004, 32 individual securities were in an unrealized loss position. As of December 31, 2003, 16 individual securities were in an unrealized loss position.
Investment Grade Debt Securities. Our investments in investment grade debt securities consist primarily of investments in federal agency securities and corporate bonds and notes. The unrealized losses on our investments in investment grade debt securities were caused by interest rate increases. Due to the fact that the decline in market value is attributable to changes in interest rates and not credit quality, and because the severity and duration of the unrealized losses were not significant, we considered these unrealized losses to be temporary at December 31, 2004.
50
5. PROPERTY AND EQUIPMENT
Property and equipment consists of the following (in thousands):
| |
Years Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2004 |
2003 |
|||||
| Laboratory and office equipment | $ | 13,804 | $ | 17,448 | |||
| Construction in progress | 288 | — | |||||
| Total property and equipment | 14,092 | 17,448 | |||||
| Less accumulated depreciation and amortization | (11,279 | ) | (13,904 | ) | |||
| Property and equipment, net | $ | 2,813 | $ | 3,544 | |||
During 2004, we wrote-off $4,900,000 of assets at their original acquisition cost and related accumulated depreciation of 4,870,000 for assets that are no longer in use. We recorded a loss on disposal of property and equipment of $30,000.
At December 31, 2004 and 2003, equipment under capital leases was approximately 5.9 million and $8.7 million, respectively. Depreciation expense was $2.2 million, $2.6 million, and $3.2 million for the years ended December 31, 2004, 2003 and 2002, respectively. No amortization expense was incurred for the year ended December 31, 2004. Amortization expense was $24,000 and $1,710,000 for the years ended December 31, 2003 and 2002, respectively.
6. LONG-TERM OBLIGATIONS
At December 31, 2004, future minimum lease payments and obligations under all noncancelable leases were as follows (in thousands):
| |
Capital Leases |
Operating Leases |
||||
|---|---|---|---|---|---|---|
| 2005 | $ | 1,452 | $ | 12,354 | ||
| 2006 | 576 | 12,795 | ||||
| 2007 | 258 | 13,512 | ||||
| 2008 | — | 13,945 | ||||
| 2009 | — | 14,399 | ||||
| 2010 and thereafter | — | 114,630 | ||||
| Total minimum payments required | 2,286 | $ | 181,635 | |||
| Less amount representing interest | (184 | ) | ||||
| Present value of future lease payments | 2,102 | |||||
| Less current portion | (1,321 | ) | ||||
| Noncurrent obligations under capital leases | $ | 781 | ||||
Our current facilities were constructed as a build-to-suit facility. Under the original lease for this new facility we were obligated to fund approximately $18.0 million of the total tenant improvements. In October 2002 we amended this original lease agreement, prior to the completion of the construction of the facility and commencement of the lease to provide for a delay of the rent commencement date until February 1, 2003 and an increase in the tenant improvement allowance from the lessor to cover all the tenant improvement construction obligations on the facility. The landlord owns 100% of these
51
tenant improvements. The lease was also amended to increase the future rental commitments to compensate for the delay of the rent commencement and the increase in the tenant improvement allowance. Since the amendment was considered a material change to the original lease, we revisited the proper accounting treatment for this lease per FAS 13 and again determined the lease to be an operating lease. We moved into the new facilities during February 2003. During May 2004, we initiated a sublease of approximately 15,000 square feet of our premises to a tenant for a period of two years. The operating minimum lease payments above are reflective of the new sublease income stream. On January 31, 2005 we entered into another amendment with the landlord to decrease the contractual rental commitments in 2005 by approximately $1.0 million. The operating lease payment schedule above reflects this amendment.
Rent expense under all operating leases amounted to approximately $14.0 million, $13.5 million and $1.9 million for the years ended December 31, 2004, 2003 and 2002, respectively.
In August 2000, we entered into an equipment lease line agreement for an aggregate total of $5.0 million. We only utilized $4.1 million of the facility before the drawdown period expired. The lease period was for four years. The interest on the lease is fixed at the time of the draw down with the interest rates ranging from 10.6% to 14.6%. This line has a bargain purchase buyout provision of $1.0. As of December 31, 2004, the remaining principal balance was approximately $145,000.
In January 2002, we entered into an additional equipment lease line agreement for an aggregate total of $2.0 million. This line was fully utilized in August 2002. The lease period was for 37 months. The interest on the lease is fixed at the time of the draw down with the interest rates ranging from 11.5% to 11.7%. This line has a buyout provision of approximately 10% of the original utilized line amount. As of December 31, 2004, the remaining principal balance was approximately $452,000.
In December 2002, we entered into an additional equipment lease line agreement for an aggregate total of $2.0 million. We originally had the ability to draw down on this line until December 2003, but this date was extended to March 2004 through an agreement reached in 2003. Only $1.4 million of this line was utilized before the drawdown period expired. The lease period is for three years. The interest on the lease is fixed at the time of any draw down and equates to approximately 10.5%. This line has a buyout provision of either the fair-market value of the equipment or 10% of the original utilized line amount. As of December 31, 2004, the remaining principal balance was approximately $778,000.
In April 2004, our equipment credit line under an original master agreement originally signed in August 2000 was extended to create an additional total borrowing limit of $2.0 million. As of December 31, 2004, $755,000 of this extended amount has been utilized. We have the ability to draw down through March 2005. The lease period will be for three years. The interest rate on the lease is fixed at drawdown and ranges from 9.2% to 9.9%. Each line has a bargain purchase buyout provision of $101. As of December 31, 2004, the remaining principal balance was approximately $727,000.
Obligations under all leases are secured by the assets financed under the leases.
7. STOCKHOLDERS' EQUITY
Common stock
In January 2002, we issued 777,778 shares of common stock in a registered direct offering to certain institutional investors at a price of $40.50 per share under a shelf registration statement. We received net proceeds of approximately $29.4 million after deducting commissions and offering costs. In
52
February 2002, we issued 51,678 shares of common stock in a registered direct offering to a certain institutional investor at a price of $38.70 per share under a shelf registration statement. We received net proceeds of approximately $1.8 million after deducting commissions and offering costs.
In June 2003, we completed a private placement with net proceeds of $45.0 million led by MPM Capital and included Frazier Healthcare, Alta Partners and HBM BioVentures. In the private placement, we issued 7,986,110 shares of our common stock at a price of $5.76 per share plus warrants (described below).
In June 2003, we initiated a rights offering pursuant to which non-transferable rights to purchase up to an aggregate of 1,736,111 shares of our common stock at a purchase price of $5.76 per share were offered to our stockholders of record as of April 29, 2003, other than certain stockholders affiliated with the investors in the private placement completed on June 26, 2003. Each such stockholder of record received one basic subscription right to purchase 0.4508 of a share of Rigel common stock at $5.76 per share for each share owned as of the record date. By July 25, 2003, the expiration of the rights offering period, our stockholders had elected to purchase an aggregate of 1,616,705 shares of our common stock for net proceeds to us of $9.1 million. The shares were issued to the participating stockholders on July 31, 2003.
In February 2004, we completed a follow-on offering in which we sold 2,850,000 shares and selling stockholders sold 315,000 shares of our common stock at a price to the public of $20.00 per share. In March 2004, the underwriters of the follow-on offering exercised their option to purchase an additional 316,750 shares of our common stock, to cover over-allotments. A total of 3,481,750 shares of our common stock were sold in the offering, of which 3,135,075 were sold by us and 346,675 were sold by selling stockholders. We received net proceeds of approximately $58.3 million from the sale of shares offered by us, net of underwriting discounts and commissions and related expenses. We did not receive any proceeds from the sale of shares by the selling stockholders.
Warrants
In conjunction with the facilities lease entered into in June 1998, we issued three warrants to the lessor to purchase an aggregate of 16,666 shares of common stock at an exercise price of $10.26 per share. The warrants are outstanding as of December 31, 2004 and are exercisable at any time up to November 28, 2007, the seventh anniversary of the closing of our initial public offering.
In conjunction with the facilities lease entered into in May 2001, we issued a warrant to the lessor to purchase 16,666 shares of our common stock at an exercise price of $80.21 per share, a 15% premium to market at the time of issuance. This warrant was outstanding as of December 31, 2004 and will expire on May 16, 2006. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $683,000. This amount has been capitalized in other long-term assets and is being amortized into expense over the life of the lease. As of December 31, 2004, approximately $595,000 remained to be amortized over the life of the lease.
In conjunction with the equipment lease line executed in January 2002, we issued a warrant to the lender to purchase 2,645 shares of our common stock at an exercise price of $37.80 per share. This warrant was outstanding as of December 31, 2004 and will expire on January 31, 2007. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $66,000. This amount has been capitalized in other long-term assets and is being amortized into expense over
53
the payment period of the equipment lease line. As of December 31, 2004, approximately $49,000 remained to be amortized over the life of the lease.
In conjunction with the equipment lease line executed in July 2002, we issued a warrant to the lender to purchase 15,432 shares of our common stock at an exercise price of $24.30 per share. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $251,000. This amount was completely expensed in 2002 in conjunction with the termination of the line. These warrants were net exercised in 2004 and resulted in the issuance of 1,086 shares of common stock.
In conjunction with the amendment of our master lease agreement for our 1180 Veterans Blvd. facility entered into in October 2002, we issued a warrant to the lessor to purchase 55,555 shares of our common stock at an exercise price of $17.73 per share. This warrant was outstanding as of December 31, 2004 and will expire on October 18, 2007. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $565,000. This amount has been capitalized in other long-term assets and is being amortized into expense over the life of the lease. As of December 31, 2004, approximately $493,000 remained to be amortized over the life of the lease.
In conjunction with the equipment lease line executed in December 2002, we issued a warrant to the lender to purchase 20,768 shares of our common stock at an exercise price of $9.63 per share. The fair market value of this warrant, as determined by the Black-Scholes valuation model, was approximately $136,000. This amount has been capitalized in other long-term assets and is being amortized into expense over the payment period of the equipment lease line. These warrants were net exercised in 2004 and resulted in the issuance of 11,343 shares of common stock.
In conjunction with the financing completed in June 2003, we issued warrants to the investors to purchase an additional 1,597,221 shares of our common stock at an exercise price of $5.76 per share. The fair value of these warrants, as determined by the Black-Scholes valuation model, was approximately $11.0 million. This amount has been allocated within "Additional paid-in capital" in our financial statements. A total of 1,041,666 of these warrants were cash exercised in 2004 resulting in proceeds to us of approximately $6.0 million. The remaining 555,555 warrant shares were net exercised in 2004 and resulted in the issuance of 415,687 shares of common stock.
Stock option plans
In January 2000, we adopted the 2000 Equity Incentive Plan, or 2000 Plan, which was approved in March 2000 by our stockholders. The 2000 Plan is an amendment and restatement of the 1997 Stock Option Plan. Under the 2000 Plan, incentive stock options, nonstatutory stock options and shares of common stock may be granted to our employees, directors and consultants. In July 2001, we adopted the 2001 Non-Officer Equity Incentive Plan, or 2001 Plan. Under the 2001 Plan, which was not approved by our stockholders, nonstatutory stock options may be granted to our employees and consultants. In April 2003, our board of directors approved an amendment to the 2000 Plan, which was subsequently approved by our stockholders at our annual meeting in June 2003, to (i) merge the 2001 Plan into the 2000 Plan and to terminate the 2001 Plan, (ii) increase the number of shares authorized for issuance under the 2000 Plan (including the available reserve from the merging of the 2001 Plan) by 1,600,000 shares of common stock, and (iii) add an evergreen feature that provides for automatic annual increases in the total number of shares reserved for issuance under the 2000 Plan. Options originally granted under our 2000 Plan and 2001 Plan expire no later than ten years from the date of grant. The option price of each incentive stock option shall be at least 100% of the fair value on the
54
date of grant, and the option price for each nonstatutory stock option shall be not less than 85% of the fair value on the date of grant, as determined by the board of directors. Options may be granted with different vesting terms from time to time, not to exceed five years from the date of grant. As of December 31, 2004, a total of 414,872 shares of common stock are authorized for issuance under the 2000 Plan.
In August 2000, we adopted the 2000 Non-Employee Directors Stock Option Plan, or Directors' Plan, which was approved in September 2000 by our stockholders. Under the original plan, each non-employee director who becomes a director of Rigel would be automatically granted a nonstatutory stock option to purchase 2,222 shares of common stock on the date on which such person first becomes a director. At each board meeting immediately following each annual meeting of stockholders, beginning with the board meeting following the 2001 Annual Stockholders' Meeting, each non-employee director would automatically be granted a nonstatutory option to purchase 556 shares of common stock. In April 2003, our board of directors approved an amendment to the Directors' Plan, which was subsequently approved by our stockholders at our annual meeting in June 2003, to (i) increase the number of shares authorized for issuance under the Directors' Plan by 66,667 shares of common stock, (ii) increase the size of the initial grants to 6,667 shares of common stock, and (iii) increase the size of the annual grant to 1,667 shares of common stock. The exercise price of options under the Directors' Plan will be equal to the fair market value of the common stock on the date of grant. The maximum term of the options granted under the Directors' Plan is ten years. All grants under the Directors' Plan will vest monthly over two years from date of grant. The Directors' Plan will terminate in September 2009, unless terminated earlier in accordance with the provisions of the Directors' Plan. As of December 31, 2004, a total of 68,177 shares of common stock are authorized for issuance under the Directors' Plan.
In June 2003, we initiated an offer to exchange options to purchase shares of our common stock with exercise prices equal to or greater than $9.00 per share currently outstanding under the 2000 Plan, the 2001 Plan and the Directors' Plan, for replacement options to purchase shares of our common stock to be granted under the 2000 Plan. There were outstanding eligible options to purchase an aggregate of 367,961 shares of our common stock as of June 26, 2003. Only officers, employees not on certain leaves of absence, consultants and non-employee members of our board of directors as of June 27, 2003, who continued to be employed or providing services through the offer expiration date of July 25, 2003, were eligible to participate in the offer. We offered to conduct the exchange with respect to eligible options on a one-for-one basis. On July 28, 2003, we accepted for cancellation options to purchase an aggregate of 344,207 shares of our common stock. On July 28, 2003, we granted replacement options to purchase an aggregate of 344,207 shares of our common stock at an exercise price of $9.20 per share, the fair market value on the date of the grant. Subject to the continuation of the optionholders' employment, service as a consultant or service as a non-employee member of our board of directors, the replacement options will vest as follows: one-fifth of the shares covered by the replacement options will vest on the six-month anniversary of the date of grant; one-fifth of the shares covered by the replacement options will vest on the twelve-month anniversary of the date of grant; and three-fifths of the shares covered by the replacement options will vest in 24 equal monthly installments over the following two years. The replacement options will expire, at the latest, on the day three years and five business days after the date of grant (if they have not been forfeited earlier due to the optionholders' termination of employment, service as a consultant or service as a non-employee member of our board of directors). All replacement options, as well as the eligible options that were not surrendered under the original offer to exchange, are being treated for financial reporting purposes
55
as variable awards. Therefore, we are recording a non-cash charge, generally for the intrinsic value of the options as they vest using the graded vesting method, reflecting increases and decreases (down to, but not below, the exercise price) in the price of our common stock in compensation expense in connection with the replacement options and the eligible options that were not exchanged. We expect to continue to reflect increases and decreases in the price of our common stock in our statement of operations with respect to these options until they are exercised, forfeited or terminated. The higher the market value of our common stock, the greater the compensation expense. For the year ended December 31, 2004, we recorded a non-cash compensation charge of $1.9 million related to all options eligible for the replacement until the options are exercised, forfeited, or terminated. For the year ended December 31, 2003, we recorded a non-cash compensation charge of $1.1 million related to all options eligible for the replacement. We are currently evaluating option valuation methodologies and assumptions in light of SFAS 123R related to our employee stock option and employee stock purchase plans.
Activity under all of the option plans through December 31, 2004 was as follows:
| |
Shares Available For Grant |
Number of Options |
Weighted-Average Exercise Price |
|||||
|---|---|---|---|---|---|---|---|---|
| Outstanding at January 1, 2002 | 521,042 | 640,119 | $ | 31.32 | ||||
| Granted | (184,768 | ) | 184,768 | $ | 30.15 | |||
| Exercised | — | (36,760 | ) | $ | 2.34 | |||
| Cancelled | 69,783 | (69,783 | ) | $ | 44.31 | |||
| Outstanding at December 31, 2002 | 406,057 | 718,344 | $ | 31.23 | ||||
| Authorized for grant | 1,962,689 | — | ||||||
| Granted | (1,991,162 | ) | 1,991,162 | $ | 8.56 | |||
| Exercised | — | (111,692 | ) | $ | 2.02 | |||
| Cancelled | 516,595 | (516,595 | ) | $ | 42.53 | |||
| Outstanding at December 31, 2003 | 894,179 | 2,081,219 | $ | 8.31 | ||||
| Authorized for grant | 392,159 | — | ||||||
| Granted | (840,327 | ) | 840,327 | $ | 20.42 | |||
| Exercised | — | (134,363 | ) | $ | 7.93 | |||
| Cancelled | 37,038 | (37,038 | ) | $ | 15.11 | |||
| Outstanding at December 31, 2004 | 483,049 | 2,750,145 | $ | 11.94 | ||||
| Exercisable at December 31, 2004 | 954,412 | $ | 10.72 | |||||
| Exercisable at December 31, 2003 | 293,164 | $ | 6.77 | |||||
| Exercisable at December 31, 2002 | 380,292 | $ | 29.88 | |||||
| Weighted average fair value of options granted during 2004 | $ | 13.79 | ||||||
| Weighted average fair value of options granted during 2003 | $ | 7.21 | ||||||
| Weighted average fair value of options granted during 2002 | $ | 20.43 | ||||||
56
Details of our stock options by exercise price is as follows:
| |
Options Outstanding |
|
Options Exercisable |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise Price |
Number of Outstanding Options |
Weighted-Average Remaining Contractual Life |
Weighted-Average Exercise Price |
Number of Options |
Weighted-Average Exercise Price |
|||||||
| $0.90 - $6.57 | 112,666 | 4.24 | $ | 1.98 | 111,841 | $ | 1.95 | |||||
| $8.15 - $9.20 | 1,755,865 | 7.42 | $ | 8.38 | 614,372 | $ | 8.44 | |||||
| $14.22 - $17.66 | 443,406 | 9.35 | $ | 17.24 | 105,781 | $ | 17.33 | |||||
| $18.35 - $22.54 | 28,045 | 9.23 | $ | 19.35 | 3,300 | $ | 22.23 | |||||
| $23.00 - $25.36 | 402,675 | 9.14 | $ | 23.22 | 111,885 | $ | 23.17 | |||||
| $37.80 - $41.58 | 6,227 | 5.57 | $ | 40.33 | 5,973 | $ | 40.28 | |||||
| $73.53 - $82.13 | 1,261 | 6.47 | $ | 74.45 | 1,260 | $ | 74.45 | |||||
| $0.90 - $82.13 | 2,750,145 | 7.86 | $ | 11.94 | 954,412 | $ | 10.72 | |||||
We granted 30,000 shares of common stock options to consultants for services in 2004. We granted 100 shares of common stock options to consultants for services in 2003. We also cancelled and regranted 16,636 common stock options to consultants in association with the repricing on July 28, 2003. We granted 7,222 common stock options to consultants in exchange for services in 2002. We recognized stock-based compensation for revaluation of consultant options of $0.4 million and $0.2 million for the years ended December 31, 2004 and 2003, respectively. We recognized stock-based compensation recovery for revaluation of consultant options of $0.2 million for the year ended December 31, 2002.
We recorded $58,000 of deferred stock compensation for the year ended December 31, 2004. We recorded no deferred stock compensation with respect to options granted to employees for the years ended December 31, 2003 and 2002. The amount recorded in 2004 related to an option grant to a certain executive officer that was modified to reduce the vesting term. This amount has been reflected as a component of stockholders' equity, and the deferred expense is being amortized to operations over the remaining vesting period of the options using the graded vesting method. As a result of our reduction in force on January 31, 2003, we recognized approximately $599,000 of stock-based compensation recovery associated with the unvested and cancelled options of the terminated employees which had previously been recognized under the graded vesting method of deferred compensation amortization. We amortized deferred stock compensation of $0.1 million, $0.6 million and $1.7 million for the years ended December 31, 2004, 2003 and 2002, respectively. At December 31, 2004, we had a total of $56,000 remaining to be amortized over the remaining vesting periods of the stock options.
2000 employee stock purchase plan
In August 2000, we adopted the 2000 Employee Stock Purchase Plan, or Purchase Plan, which was approved in September 2000 by our stockholders. In April 2003, our board of directors approved an amendment to the Purchase Plan, which was subsequently approved by our stockholders at our annual meeting in June 2003, to (i) increase the number of shares authorized for issuance under the Purchase Plan by 66,667 shares of common stock, and (ii) change the evergreen feature of the plan. The amendment provides that the increase in the number of shares reserved automatically pursuant to the evergreen feature will be equal to the least of 1% of the outstanding shares on the date of the annual increase, 88,889 shares or such amount as may be determined by the board. The Purchase Plan permits eligible employees to purchase common stock at a discount through payroll deductions during defined
57
offering periods. The price at which the stock is purchased is equal to the lower of 85% of the fair market value of the common stock on the first day of the offering or 85% of the fair market value of our common stock on the purchase date. The initial offering period commenced on the effective date of our initial public offering. We issued 93,529, 37,112 and 19,273 shares of common stock during 2004, 2003 and 2002, respectively, pursuant to the Purchase Plan at an average price of $8.76 per share, $8.45 per share, and $18.63 per share in 2004, 2003 and 2002, respectively. For 2004, 2003 and 2002, the weighted average fair value of stock issued under the Purchase Plan was $6.55, $4.33 and $1.68, respectively. A total of 44,444 shares of our common stock were initially reserved for issuance under the Purchase Plan. The Purchase Plan provides for annual increases in the number of shares available for issuance under the Purchase Plan on each anniversary date of the effective date of the offering. The number of shares reserved for future issuance under the Purchase Plan was increased by 88,888, 66,667 and 44,444 during 2004, 2003 and 2002, respectively.
Reserved shares
As of December 31, 2004, we had reserved shares of common stock for future issuance as follows:
| |
December 31, 2004 |
||
|---|---|---|---|
| Warrants | 91,532 | ||
| Incentive stock plans. | 3,233,194 | ||
| Purchase Plan | 123,072 | ||
| Total | 3,447,798 | ||
8. INCOME TAXES
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred tax assets are as follows:
| |
December 31, |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
2004 |
2003 |
||||||
| Deferred tax assets | ||||||||
| Net operating loss carryforwards | $ | 60,462 | $ | 46,440 | ||||
| Research and development credits | 7,637 | 6,425 | ||||||
| Capitalized research and development expenses | 9,749 | 7,643 | ||||||
| Other, net | 9,140 | 2,417 | ||||||
| Total deferred tax assets | 86,988 | 62,925 | ||||||
| Valuation allowance | (86,988 | ) | (62,925 | ) | ||||
| Net deferred tax assets | $ | — | $ | — | ||||
Realization of deferred tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance. The valuation allowance increased by $24.1 million, $18.5 million and $18.2 million during 2004, 2003 and 2002, respectively.
58
Included in the valuation allowance balance at December 31, 2004 is $1.6 million related to the exercise of stock options which are not reflected as an expense for financial reporting purposes. Accordingly, any future reduction in the valuation allowance relating to this amount will be credited directly to equity and not reflected as an income tax benefit in the statement of operations.
As of December 31, 2004, we had net operating loss carryforwards for federal income tax purposes of approximately $175.9 million, which expire beginning in the year 2011 and federal research and development tax credits of approximately $11.2 million, which will begin to expire in 2013.
Utilization of the net operating loss and credit may be subject to a substantial annual limitation due to the "change in ownership" provisions of the Internal Revenue Code of 1986 (IRC) and similar state provisions. The annual limitation may result in the expiration of net operating losses and credits before utilization.
9. SELECTED QUARTERLY FINANCIAL DATA (unaudited, in thousands, except per share amounts)
| |
Year Ended December 31, 2004 |
Year Ended December 31, 2003 |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Q1 |
Q2 |
Q3 |
Q4 |
Q1 |
Q2 |
Q3 |
Q4 |
|||||||||||||||||
| Revenue | $ | 1,487 | $ | 1,487 | $ | 659 | $ | 1,100 | $ | 4,497 | $ | 2,349 | $ | 2,103 | $ | 2,106 | |||||||||
| Net loss | $ | (13,051 | ) | $ | (12,342 | ) | $ | (16,136 | ) | $ | (14,726 | ) | $ | (7,800 | ) | $ | (10,466 | ) | $ | (11,069 | ) | $ | (11,862 | ) | |
| Net loss per common share, basic and diluted | $ | (0.81 | ) | $ | (0.68 | ) | $ | (0.88 | ) | $ | (0.75 | ) | $ | (1.53 | ) | $ | (1.90 | ) | $ | (0.78 | ) | $ | (0.80 | ) | |
| Weighted average shares used in computing net loss per common share, basic and diluted | 16,047 | 18,215 | 18,386 | 19,544 | 5,089 | 5,496 | 14,224 | 14,796 | |||||||||||||||||
59
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) promulgated under the Securities Exchange Act of 1934, as amended (the Exchange Act). Based on this evaluation, our principal executive officer and our principal financial officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this annual report.
Management's Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f). Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control—Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2004.
Report of Independent Registered Public Accounting Firm on Internal Control over Financial Reporting
The Board of Directors and Stockholders Rigel Pharmaceuticals, Inc.
We have audited management's assessment, included in the accompanying Management's Report on Internal Control Over Financial Reporting appearing under Item 9A, that Rigel Pharmaceuticals, Inc. maintained effective internal control over financial reporting as of December 31, 2004, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Rigel Pharmaceutical, Inc.'s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting. Our responsibility is to express an opinion on management's assessment and an opinion on the effectiveness of the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, evaluating management's assessment, testing and evaluating the design and operating effectiveness of internal control, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are
60
recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, management's assessment that Rigel Pharmaceuticals, Inc. maintained effective internal control over financial reporting as of December 31, 2004, is fairly stated, in all material respects, based on the COSO criteria. Also, in our opinion, Rigel Pharmaceuticals, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2004, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of Rigel Pharmaceutical, Inc. as of December 31, 2004 and 2003, and the related statements of operations, stockholders' equity, and cash flows for each of the three years in the period ended December 31, 2004 of Rigel Pharmaceuticals, Inc. and our report dated March 10, 2005 expressed an unqualified opinion thereon.
| /s/ ERNST & YOUNG LLP |
Palo
Alto, California
March 10, 2004
Changes in Internal Controls over Financial Reporting
There were no changes in our internal controls over financial reporting that occurred during our most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
61
Item 10. Directors and Executive Officers of the Registrant
Information regarding directors and executive officers is incorporated by reference to the information set forth under the caption "Directors and Executive Officers" in our Proxy Statement for the Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission on or prior to April 30, 2005.
In 2003, we adopted a code of ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. Our code of ethics is on our website at http://www.rigel.com/pdf/codeofconduct.pdf with "Investor Resources" materials. If we make any substantive amendments to the code or grant any waiver from a provision of the code applicable to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on a Form 8-K.
Item 11. Executive Compensation
Information regarding executive compensation is incorporated by reference to the information set forth under the caption "Executive Compensation" in our Proxy Statement for the Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission on or prior to April 30, 2005.
Item 12. Security Ownership of Certain Beneficial Owners and Management
Information regarding security ownership of certain beneficial owners and management is incorporated by reference to the information set forth under the caption "Security Ownership of Certain Beneficial Owners and Management" and "Equity Compensation Plan Information" in our Proxy Statement for the Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission on or around April 30, 2005.
Item 13. Certain Relationships and Related Transactions
Information regarding certain relationships and related transactions is incorporated by reference to the information set forth under the caption "Certain Transactions" in our Proxy Statement for the Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission on or prior to April 30, 2005.
Item 14. Principal Accounting Fees and Services.
Information regarding principal accounting fees and services is incorporated by reference to the information set forth under the caption "Ratification of Independent Auditors" in our Proxy Statement for the Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission on or prior to April 30, 2005.
62
Item 15. Exhibits, Financial Statement Schedules
| 3.1(1) | Amended and Restated Certificate of Incorporation. | |
| 3.2(2) | Amended and Restated Bylaws. | |
| 4.1(1) | Specimen Common Stock Certificate. | |
| 4.3(2) | Form of warrant to purchase shares of common stock. | |
| 4.7(3) | Amended and Restated Warrant issued to Kwacker Limited for the purchase of shares of common stock. | |
| 4.8(11) | Warrant issued to TBCC Funding Trust II for the purchase of shares of common stock. | |
| 4.10(3) | Warrant issued to Kwacker Limited for the purchase of shares of common stock. | |
| 4.23(13) | Second Investor Rights Agreement between Rigel and certain investors, dated June 26, 2003. | |
| 10.1(2) | Form of Indemnity Agreement. | |
| 10.2(4)(5) | 2000 Equity Incentive Plan, as amended. | |
| 10.3(2)(4) | Form of Stock Option Agreement pursuant to 2000 Equity Incentive Plan. | |
| 10.4(4)(5) | 2000 Employee Stock Purchase Plan, as amended. | |
| 10.5(4)(5) | 2000 Non-Employee Directors' Stock Option Plan, as amended. | |
| 10.6(2) | Collaboration Agreement between Rigel and Janssen Pharmaceutica N.V., dated December 4, 1998. | |
| 10.7(2) | Collaborative Research and License Agreement between Rigel and Pfizer Inc., dated January 31, 1999. | |
| 10.8(2) | Collaboration Agreement between Rigel and Novartis Pharma AG, dated May 26, 1999. | |
| 10.9(2)(6) | License and Research Agreement between Rigel and Cell Genesys, Inc., dated September 2, 1999. | |
| 10.10(2) | Collaborative Research and Development Agreement between Rigel and Neurocrine Biosciences, Inc., dated December 1997. | |
| 10.11(2)(4) | Employment Agreement between Rigel and Donald Payan, dated January 16, 1997. | |
| 10.13(2) | Technology Transfer Agreement between Rigel and Questcor Pharmaceuticals, Inc., dated September 22, 2000. | |
| 10.14(6)(7) | License and Research Agreement (Amended and Restated) between Rigel and Cell Genesys, Inc., dated September 2, 1999, as amended and restated on March 26, 2001. | |
| 10.15(8) | Lease termination agreement between Rigel and Britannia Pointe Grand Limited Partnership, dated May 6, 2001. | |
| 10.16(8) | Build-to-suit lease between Rigel and Slough BTC, LLC, dated May 16, 2001. | |
| 10.17(8) | First Amendment to the Collaboration Agreement between Rigel and Novartis Pharma AG, dated May 18, 2001. | |
| 10.18(6)(9) | Second Amendment to the Collaboration Agreement between Rigel and Novartis Pharma AG, dated July 6, 2001. | |
| 10.19(6)(9) | Second Amendment, dated July 1, 2001, to the Collaboration Agreement between Rigel and Cell Genesys, Inc. | |
63
| 10.22(10) | First Amendment to the Collaboration Agreement by and between Rigel and Janssen Pharmaceutical N.V., dated June 30, 2000. | |
| 10.23(10) | Second Amendment to the Collaboration Agreement by and between Rigel and Janssen Pharmaceutical N.V., dated December 4, 2001. | |
| 10.24(12) | Loan and Security Agreement between Rigel and Comerica Bank — California, dated July 12, 2002. | |
| 10.25(6)(12) | Collaboration Agreement between Rigel and Daiichi Pharmaceutical Co., Ltd., dated August 1, 2002. | |
| 10.26(3)(6) | Amendment to Build-to-suit lease between Rigel and Slough BTC, LLC, dated October 18, 2002. | |
| 10.27(3) | Master Lease Agreement between Rigel and Lighthouse Capital Partners IV, L.P., dated December 23, 2002. | |
| 10.28(3)(4) | Employment Agreement between Rigel and Elliott B. Grossbard, dated as of March 18, 2002. | |
| 10.29(3)(4) | Amendment to Employment Agreement, between Rigel and Donald Payan, dated as of March 5, 2003. | |
| 23.1(14) | Consent of Ernst & Young LLP, Independent Auditors. | |
| 24.1 | Power of Attorney (included on signature page). | |
| 31.1(14) | Certification required by Rule 13a-14(a) or Rule 15d-14(a) | |
| 31.2(14) | Certification required by Rule 13a-14(a) or Rule 15d-14(a) | |
| 32.1(15) | Certification required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. 1350). |
64
65
Pursuant to the requirements of the Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of South San Francisco, State of California, on March 16, 2005.
| Rigel Pharmaceuticals, Inc. | |||
By: |
/s/ JAMES M. GOWER James M. Gower Chairman of the Board and Chief Executive Officer |
||
By: |
/s/ JAMES H. WELCH James H. Welch Vice President, Chief Financial Officer and Secretary |
||
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints James M. Gower and James H. Welch, and each of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him and in his name, place, and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming that all said attorneys-in-fact and agents, or any of them or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated:
| Signature |
Title |
Date |
||
|---|---|---|---|---|
/s/ JAMES M. GOWER James M. Gower |
Chairman of the Board, Chief Executive Officer and Director (Principal Executive Officer) |
March 16, 2005 |
||
/s/ JAMES H. WELCH James H. Welch |
Vice President, Chief Financial Officer, and Secretary (Principal Finance and Accounting Officer) |
March 16, 2005 |
||
/s/ DONALD G. PAYAN Donald G. Payan |
Executive Vice President, Chief Scientific Officer and Director |
March 16, 2005 |
||
/s/ JEAN DELEAGE Jean Deleage |
Director |
March 16, 2005 |
||
66
/s/ ALAN D. FRAZIER Alan D. Frazier |
Director |
March 16, 2005 |
||
/s/ PETER S. RINGROSE Peter S. Ringrose |
Director |
March 16, 2005 |
||
/s/ WALTER H. MOOS Walter H. Moos |
Director |
March 16, 2005 |
||
/s/ HOLLINGS C. RENTON Hollings C. Renton |
Director |
March 16, 2005 |
||
/s/ STEPHEN A. SHERWIN Stephen A. Sherwin |
Director |
March 16, 2005 |
||
/s/ NICHOLAS J. SIMON, III Nicholas J. Simon, III |
Director |
March 16, 2005 |
67
| 3.1(1) | Amended and Restated Certificate of Incorporation. | |
| 3.2(2) | Amended and Restated Bylaws. | |
| 4.1(1) | Specimen Common Stock Certificate. | |
| 4.3(2) | Form of warrant to purchase shares of common stock. | |
| 4.7(3) | Amended and Restated Warrant issued to Kwacker Limited for the purchase of shares of common stock. | |
| 4.8(11) | Warrant issued to TBCC Funding Trust II for the purchase of shares of common stock. | |
| 4.10(3) | Warrant issued to Kwacker Limited for the purchase of shares of common stock. | |
| 4.23(13) | Second Investor Rights Agreement between Rigel and certain investors, dated June 26, 2003. | |
| 10.1(2) | Form of Indemnity Agreement. | |
| 10.2(4)(5) | 2000 Equity Incentive Plan, as amended. | |
| 10.3(2)(4) | Form of Stock Option Agreement pursuant to 2000 Equity Incentive Plan. | |
| 10.4(4)(5) | 2000 Employee Stock Purchase Plan, as amended. | |
| 10.5(4)(5) | 2000 Non-Employee Directors' Stock Option Plan, as amended. | |
| 10.6(2) | Collaboration Agreement between Rigel and Janssen Pharmaceutica N.V., dated December 4, 1998. | |
| 10.7(2) | Collaborative Research and License Agreement between Rigel and Pfizer Inc., dated January 31, 1999. | |
| 10.8(2) | Collaboration Agreement between Rigel and Novartis Pharma AG, dated May 26, 1999. | |
| 10.9(2)(6) | License and Research Agreement between Rigel and Cell Genesys, Inc., dated September 2, 1999. | |
| 10.10(2) | Collaborative Research and Development Agreement between Rigel and Neurocrine Biosciences, Inc., dated December 1997. | |
| 10.11(2)(4) | Employment Agreement between Rigel and Donald Payan, dated January 16, 1997. | |
| 10.13(2) | Technology Transfer Agreement between Rigel and Questcor Pharmaceuticals, Inc., dated September 22, 2000. | |
| 10.14(6)(7) | License and Research Agreement (Amended and Restated) between Rigel and Cell Genesys, Inc., dated September 2, 1999, as amended and restated on March 26, 2001. | |
| 10.15(8) | Lease termination agreement between Rigel and Britannia Pointe Grand Limited Partnership, dated May 6, 2001. | |
| 10.16(8) | Build-to-suit lease between Rigel and Slough BTC, LLC, dated May 16, 2001. | |
| 10.17(8) | First Amendment to the Collaboration Agreement between Rigel and Novartis Pharma AG, dated May 18, 2001. | |
| 10.18(6)(9) | Second Amendment to the Collaboration Agreement between Rigel and Novartis Pharma AG, dated July 6, 2001. | |
| 10.19(6)(9) | Second Amendment, dated July 1, 2001, to the Collaboration Agreement between Rigel and Cell Genesys, Inc. | |
| 10.22(10) | First Amendment to the Collaboration Agreement by and between Rigel and Janssen Pharmaceutical N.V., dated June 30, 2000. | |
| 10.23(10) | Second Amendment to the Collaboration Agreement by and between Rigel and Janssen Pharmaceutical N.V., dated December 4, 2001. | |
| 10.24(12) | Loan and Security Agreement between Rigel and Comerica Bank — California, dated July 12, 2002. | |
| 10.25(6)(12) | Collaboration Agreement between Rigel and Daiichi Pharmaceutical Co., Ltd., dated August 1, 2002. | |
| 10.26(3)(6) | Amendment to Build-to-suit lease between Rigel and Slough BTC, LLC, dated October 18, 2002. | |
68
| 10.27(3) | Master Lease Agreement between Rigel and Lighthouse Capital Partners IV, L.P., dated December 23, 2002. | |
| 10.28(3)(4) | Employment Agreement between Rigel and Elliott B. Grossbard, dated as of March 18, 2002. | |
| 10.29(3)(4) | Amendment to Employment Agreement, between Rigel and Donald Payan, dated as of March 5, 2003. | |
| 23.1(14) | Consent of Ernst & Young LLP, Independent Auditors. | |
| 24.1 | Power of Attorney (included on signature page). | |
| 31.1(14) | Certification required by Rule 13a-14(a) or Rule 15d-14(a) | |
| 31.2(14) | Certification required by Rule 13a-14(a) or Rule 15d-14(a) | |
| 32.1(15) | Certification required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. 1350). |
69